A recessive founder mutation in regulator of telomere elongation helicase 1, RTEL1, underlies severe immunodeficiency and features of Hoyeraal Hreidarsson syndrome
- PMID: 24009516
- PMCID: PMC3757051
- DOI: 10.1371/journal.pgen.1003695
A recessive founder mutation in regulator of telomere elongation helicase 1, RTEL1, underlies severe immunodeficiency and features of Hoyeraal Hreidarsson syndrome
Abstract
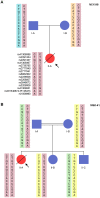
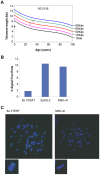
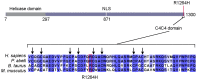
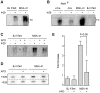
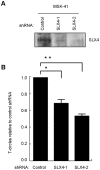
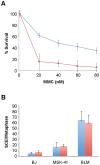
Dyskeratosis congenita (DC) is a heterogeneous inherited bone marrow failure and cancer predisposition syndrome in which germline mutations in telomere biology genes account for approximately one-half of known families. Hoyeraal Hreidarsson syndrome (HH) is a clinically severe variant of DC in which patients also have cerebellar hypoplasia and may present with severe immunodeficiency and enteropathy. We discovered a germline autosomal recessive mutation in RTEL1, a helicase with critical telomeric functions, in two unrelated families of Ashkenazi Jewish (AJ) ancestry. The affected individuals in these families are homozygous for the same mutation, R1264H, which affects three isoforms of RTEL1. Each parent was a heterozygous carrier of one mutant allele. Patient-derived cell lines revealed evidence of telomere dysfunction, including significantly decreased telomere length, telomere length heterogeneity, and the presence of extra-chromosomal circular telomeric DNA. In addition, RTEL1 mutant cells exhibited enhanced sensitivity to the interstrand cross-linking agent mitomycin C. The molecular data and the patterns of inheritance are consistent with a hypomorphic mutation in RTEL1 as the underlying basis of the clinical and cellular phenotypes. This study further implicates RTEL1 in the etiology of DC/HH and immunodeficiency, and identifies the first known homozygous autosomal recessive disease-associated mutation in RTEL1.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

