Mesenchymal Stem Cell Lines Isolated by Different Isolation Methods Show Variations in the Regulation of Graft-versus-host Disease
- PMID: 24009540
- PMCID: PMC3759710
- DOI: 10.4110/in.2013.13.4.133
Mesenchymal Stem Cell Lines Isolated by Different Isolation Methods Show Variations in the Regulation of Graft-versus-host Disease
Erratum in
- Immune Netw. 2014 Feb;14(1):66
Abstract
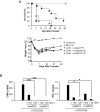
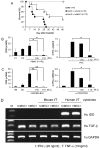
Since the discovery of the immunomodulation property of mesenchymal stem cells (MSCs) about a decade ago, it has been extensively investigated whether MSCs can be used for the treatment of immune-related diseases, such as graft-versus-host disease (GvHD). However, how to evaluate the efficacy of human MSCs for the clinical trial is still unclear. We used an MHC-mismatched model of GvHD (B6 into BALB/c). Surprisingly, the administration of the human MSCs (hMSCs) could reduce the GvHD-related mortality of the mouse recipients and xenogeneically inhibit mouse T-cell proliferation and IFN-γ production in vitro. We recently established a new protocol for the isolation of a homogeneous population of MSCs called subfractionation culturing methods (SCM), and established a library of clonal MSC lines. Therefore, we also investigated whether MSCs isolated by the conventional gradient centrifugation method (GCM) and SCM show different efficacy in vivo. Intriguingly, clonal hMSCs (hcMSCs) isolated by SCM showed better efficacy than hMSCs isolated by GCM. Based on these results, the MHC-mismatched model of GvHD may be useful for evaluating the efficacy of human MSCs before the clinical trial. The results of this study suggest that different MSC lines may show different efficacy in vivo and in vitro.
Keywords: Efficacy; IFN-γ; Mesenchymal stem cells; T-cell; graft-versus-host disease.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures



References
-
- Goker H, Haznedaroglu IC, Chao NJ. Acute graft-vs-host disease: pathobiology and management. Exp Hematol. 2001;29:259–277. - PubMed
-
- Menillo SA, Goldberg SL, McKiernan P, Pecora AL. Intraoral psoralen ultraviolet A irradiation (PUVA) treatment of refractory oral chronic graft-versus-host disease following allogeneic stem cell transplantation. Bone Marrow Transplant. 2001;28:807–808. - PubMed
-
- Rager A, Frey N, Goldstein SC, Reshef R, Hexner EO, Loren A, Luger SM, Perl A, Tsai D, Davis J, Vozniak M, Smith J, Stadtmauer EA, Porter DL. Inflammatory cytokine inhibition with combination daclizumab and infliximab for steroid-refractory acute GVHD. Bone Marrow Transplant. 2011;46:430–435. - PMC - PubMed
-
- Schub N, Günther A, Schrauder A, Claviez A, Ehlert C, Gramatzki M, Repp R. Therapy of steroid-refractory acute GVHD with CD52 antibody alemtuzumab is effective. Bone Marrow Transplant. 2011;46:143–147. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

