Low micromolar Ba(2+) potentiates glutamate transporter current in hippocampal astrocytes
- PMID: 24009556
- PMCID: PMC3755269
- DOI: 10.3389/fncel.2013.00135
Low micromolar Ba(2+) potentiates glutamate transporter current in hippocampal astrocytes
Abstract
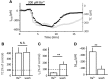
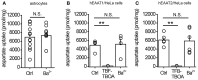
Glutamate uptake, mediated by electrogenic glutamate transporters largely localized in astrocytes, is responsible for the clearance of glutamate released during excitatory synaptic transmission. Glutamate uptake also determines the availability of glutamate for extrasynaptic glutamate receptors. The efficiency of glutamate uptake is commonly estimated from the amplitude of transporter current recorded in astrocytes. We recorded currents in voltage-clamped hippocampal CA1 stratum radiatum astrocytes in rat hippocampal slices induced by electrical stimulation of the Schaffer collaterals. A Ba(2+)-sensitive K(+) current mediated by inward rectifying potassium channels (Kir) accompanied the transporter current. Surprisingly, Ba(2+) not only suppressed the K(+) current and changed holding current (presumably, mediated by Kir) but also increased the transporter current at lower concentrations. However, Ba(2+) did not significantly increase the uptake of aspartate in cultured astrocytes, suggesting that increase in the amplitude of the transporter current does not always reflect changes in glutamate uptake.
Keywords: astrocytes; barium; glutamate transporters; glutamate uptake; hippocampus.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

