β1- and β3- voltage-gated sodium channel subunits modulate cell surface expression and glycosylation of Nav1.7 in HEK293 cells
- PMID: 24009557
- PMCID: PMC3757325
- DOI: 10.3389/fncel.2013.00137
β1- and β3- voltage-gated sodium channel subunits modulate cell surface expression and glycosylation of Nav1.7 in HEK293 cells
Abstract
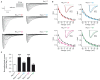
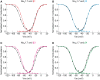
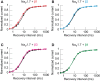
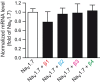
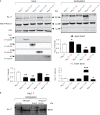
Voltage-gated sodium channels (Navs) are glycoproteins composed of a pore-forming α-subunit and associated β-subunits that regulate Nav α-subunit plasma membrane density and biophysical properties. Glycosylation of the Nav α-subunit also directly affects Navs gating. β-subunits and glycosylation thus comodulate Nav α-subunit gating. We hypothesized that β-subunits could directly influence α-subunit glycosylation. Whole-cell patch clamp of HEK293 cells revealed that both β1- and β3-subunits coexpression shifted V ½ of steady-state activation and inactivation and increased Nav1.7-mediated I Na density. Biotinylation of cell surface proteins, combined with the use of deglycosydases, confirmed that Nav1.7 α-subunits exist in multiple glycosylated states. The α-subunit intracellular fraction was found in a core-glycosylated state, migrating at ~250 kDa. At the plasma membrane, in addition to the core-glycosylated form, a fully glycosylated form of Nav1.7 (~280 kDa) was observed. This higher band shifted to an intermediate band (~260 kDa) when β1-subunits were coexpressed, suggesting that the β1-subunit promotes an alternative glycosylated form of Nav1.7. Furthermore, the β1-subunit increased the expression of this alternative glycosylated form and the β3-subunit increased the expression of the core-glycosylated form of Nav1.7. This study describes a novel role for β1- and β3-subunits in the modulation of Nav1.7 α-subunit glycosylation and cell surface expression.
Keywords: Navs β-subunits; biophysical properties; glycosylation; trafficking; voltage-gated sodium channels (Navs).
Figures

Similar articles
-
Co-expression of β Subunits with the Voltage-Gated Sodium Channel NaV1.7: the Importance of Subunit Association and Phosphorylation and Their Effects on Channel Pharmacology and Biophysics.J Mol Neurosci. 2018 Jun;65(2):154-166. doi: 10.1007/s12031-018-1082-6. Epub 2018 May 10. J Mol Neurosci. 2018. PMID: 29744740
-
Functional expression of Rat Nav1.6 voltage-gated sodium channels in HEK293 cells: modulation by the auxiliary β1 subunit.PLoS One. 2014 Jan 3;9(1):e85188. doi: 10.1371/journal.pone.0085188. eCollection 2014. PLoS One. 2014. PMID: 24404202 Free PMC article.
-
Functional modulation of the human voltage-gated sodium channel NaV1.8 by auxiliary β subunits.Channels (Austin). 2021 Dec;15(1):79-93. doi: 10.1080/19336950.2020.1860399. Channels (Austin). 2021. PMID: 33315536 Free PMC article.
-
Cell-Adhesion Properties of β-Subunits in the Regulation of Cardiomyocyte Sodium Channels.Biomolecules. 2020 Jul 1;10(7):989. doi: 10.3390/biom10070989. Biomolecules. 2020. PMID: 32630316 Free PMC article. Review.
-
New focus on cardiac voltage-gated sodium channel β1 and β1B: Novel targets for treating and understanding arrhythmias?Heart Rhythm. 2025 Jan;22(1):181-191. doi: 10.1016/j.hrthm.2024.06.029. Epub 2024 Jun 21. Heart Rhythm. 2025. PMID: 38908461 Free PMC article. Review.
Cited by
-
A Push-Pull Mechanism Between PRRT2 and β4-subunit Differentially Regulates Membrane Exposure and Biophysical Properties of NaV1.2 Sodium Channels.Mol Neurobiol. 2023 Mar;60(3):1281-1296. doi: 10.1007/s12035-022-03112-x. Epub 2022 Nov 28. Mol Neurobiol. 2023. PMID: 36441479 Free PMC article.
-
Identification and In-Silico study of non-synonymous functional SNPs in the human SCN9A gene.PLoS One. 2024 Feb 23;19(2):e0297367. doi: 10.1371/journal.pone.0297367. eCollection 2024. PLoS One. 2024. PMID: 38394191 Free PMC article.
-
Absolute Quantification of Nav1.5 Expression by Targeted Mass Spectrometry.Int J Mol Sci. 2022 Apr 10;23(8):4177. doi: 10.3390/ijms23084177. Int J Mol Sci. 2022. PMID: 35456996 Free PMC article.
-
Voltage-gated Sodium Channels and Blockers: An Overview and Where Will They Go?Curr Med Sci. 2019 Dec;39(6):863-873. doi: 10.1007/s11596-019-2117-0. Epub 2019 Dec 16. Curr Med Sci. 2019. PMID: 31845216 Review.
-
β1 subunit stabilises sodium channel Nav1.7 against mechanical stress.J Physiol. 2018 Jun;596(12):2433-2445. doi: 10.1113/JP275905. Epub 2018 May 20. J Physiol. 2018. PMID: 29659026 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

