The blood-brain barrier: an engineering perspective
- PMID: 24009582
- PMCID: PMC3757302
- DOI: 10.3389/fneng.2013.00007
The blood-brain barrier: an engineering perspective
Abstract
It has been more than 100 years since Paul Ehrlich reported that various water-soluble dyes injected into the circulation did not enter the brain. Since Ehrlich's first experiments, only a small number of molecules, such as alcohol and caffeine have been found to cross the blood-brain barrier, and this selective permeability remains the major roadblock to treatment of many central nervous system diseases. At the same time, many central nervous system diseases are associated with disruption of the blood-brain barrier that can lead to changes in permeability, modulation of immune cell transport, and trafficking of pathogens into the brain. Therefore, advances in our understanding of the structure and function of the blood-brain barrier are key to developing effective treatments for a wide range of central nervous system diseases. Over the past 10 years it has become recognized that the blood-brain barrier is a complex, dynamic system that involves biomechanical and biochemical signaling between the vascular system and the brain. Here we reconstruct the structure, function, and transport properties of the blood-brain barrier from an engineering perspective. New insight into the physics of the blood-brain barrier could ultimately lead to clinical advances in the treatment of central nervous system diseases.
Keywords: blood-brain barrier; capillary; microvasculature; neurovascular unit; transport.
Figures
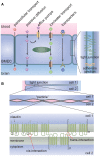
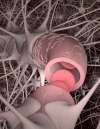

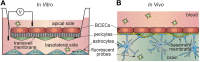

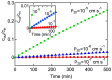


References
-
- Aiello L. C., Dunbar R. I. M. (1993). Neocortex size, group-size, and the evolution of language. Curr. Anthropol. 34, 184–193 10.1086/204160 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

