Synthetic biology of cyanobacteria: unique challenges and opportunities
- PMID: 24009604
- PMCID: PMC3755261
- DOI: 10.3389/fmicb.2013.00246
Synthetic biology of cyanobacteria: unique challenges and opportunities
Abstract
Photosynthetic organisms, and especially cyanobacteria, hold great promise as sources of renewably-produced fuels, bulk and specialty chemicals, and nutritional products. Synthetic biology tools can help unlock cyanobacteria's potential for these functions, but unfortunately tool development for these organisms has lagged behind that for S. cerevisiae and E. coli. While these organisms may in many cases be more difficult to work with as "chassis" strains for synthetic biology than certain heterotrophs, the unique advantages of autotrophs in biotechnology applications as well as the scientific importance of improved understanding of photosynthesis warrant the development of these systems into something akin to a "green E. coli." In this review, we highlight unique challenges and opportunities for development of synthetic biology approaches in cyanobacteria. We review classical and recently developed methods for constructing targeted mutants in various cyanobacterial strains, and offer perspective on what genetic tools might most greatly expand the ability to engineer new functions in such strains. Similarly, we review what genetic parts are most needed for the development of cyanobacterial synthetic biology. Finally, we highlight recent methods to construct genome-scale models of cyanobacterial metabolism and to use those models to measure properties of autotrophic metabolism. Throughout this paper, we discuss some of the unique challenges of a diurnal, autotrophic lifestyle along with how the development of synthetic biology and biotechnology in cyanobacteria must fit within those constraints.
Keywords: biofuel; cyanobacteria; flux balance analysis; metabolic flux analysis; synthetic biology; systems biology.
Figures
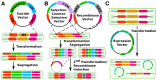
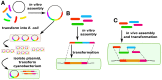
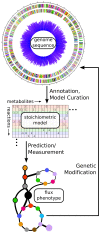
References
-
- Berto P., D'adamo S., Bergantino E., Vallese F., Giacometti G. M., Costantini P. (2011). The cyanobacterium Synechocystis sp. PCC 6803 is able to express an active [FeFe]-hydrogenase without additional maturation proteins. Biochem. Biophys. Res. Commun. 405, 678–683 10.1016/j.bbrc.2011.01.095 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

