Intraductal carcinoma of prostate: a comprehensive and concise review
- PMID: 24009625
- PMCID: PMC3759629
- DOI: 10.4132/KoreanJPathol.2013.47.4.307
Intraductal carcinoma of prostate: a comprehensive and concise review
Erratum in
- Korean J Pathol. 2013 Oct;47(5):502. Park, Yong Wok [corrected to Park, Yong Wook]
Abstract
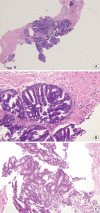
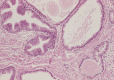
Intraductal carcinoma of the prostate (IDC-P) is defined as a proliferation of prostate adenocarcinoma cells distending and spanning the lumen of pre-existing benign prostatic ducts and acini, with at least focal preservation of basal cells. Studies demonstrate that IDC-P is strongly associated with high-grade (Gleason grades 4/5), large-volume invasive prostate cancers. In addition, recent genetic studies indicate that IDC-P represents intraductal spread of invasive carcinoma, rather than a precursor lesion. Some of the architectural patterns in IDC-P exhibit architectural overlap with one of the main differential diagnoses, high-grade prostatic intraepithelial neoplasia (HGPIN). In these instances, additional diagnostic criteria for IDC-P, including marked nuclear pleomorphism, non-focal comedonecrosis (>1 duct showing comedonecrosis), markedly distended normal ducts/acini, positive nuclear staining for ERG, and cytoplasmic loss of PTEN by immunohistochemistry, can help make the distinction. This distinction between IDC-P and HGPIN is of critical importance because IDC-P has an almost constant association with invasive carcinoma and has negative clinical implications, including shorter relapse-free survival, early biochemical relapse, and metastatic failure rate after radiotherapy. Therefore, IDC-P should be reported in prostate biopsies and radical prostatectomies, regardless of the presence of an invasive component. This article will review the history, diagnostic criteria, molecular genetics, and clinical significance of IDC-P.
Keywords: Intraductal carcinoma of the prostate; Neoplasms; Prostate.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures






Similar articles
-
Heterogeneous clinicopathological features of intraductal carcinoma of the prostate: a comparison between "precursor-like" and "regular type" lesions.Int J Clin Exp Pathol. 2014 Apr 15;7(5):2518-26. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 24966964 Free PMC article.
-
ETS gene aberrations in atypical cribriform lesions of the prostate: Implications for the distinction between intraductal carcinoma of the prostate and cribriform high-grade prostatic intraepithelial neoplasia.Am J Surg Pathol. 2010 Apr;34(4):478-85. doi: 10.1097/PAS.0b013e3181d6827b. Am J Surg Pathol. 2010. PMID: 20220513
-
Clinicopathological analysis of intraductal proliferative lesions of prostate: intraductal carcinoma of prostate, high-grade prostatic intraepithelial neoplasia, and atypical cribriform lesion.Hum Pathol. 2014 Aug;45(8):1572-81. doi: 10.1016/j.humpath.2014.03.011. Epub 2014 Apr 12. Hum Pathol. 2014. PMID: 24842280
-
Intraductal carcinoma of the prostate.Arch Pathol Lab Med. 2012 Apr;136(4):418-25. doi: 10.5858/arpa.2011-0519-RA. Arch Pathol Lab Med. 2012. PMID: 22458904 Review.
-
Intraductal carcinoma of the prostate: the whole story.Pathology. 2013 Oct;45(6):533-9. doi: 10.1097/PAT.0b013e3283653322. Pathology. 2013. PMID: 24018805 Review.
Cited by
-
Microfluidic Applications in Prostate Cancer Research.Micromachines (Basel). 2024 Sep 27;15(10):1195. doi: 10.3390/mi15101195. Micromachines (Basel). 2024. PMID: 39459070 Free PMC article. Review.
-
Comedonecrosis Revisited: Strong Association With Intraductal Carcinoma of the Prostate.Am J Surg Pathol. 2018 Aug;42(8):1036-1041. doi: 10.1097/PAS.0000000000001104. Am J Surg Pathol. 2018. PMID: 29878934 Free PMC article.
-
Heterogeneous clinicopathological features of intraductal carcinoma of the prostate: a comparison between "precursor-like" and "regular type" lesions.Int J Clin Exp Pathol. 2014 Apr 15;7(5):2518-26. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 24966964 Free PMC article.
-
[Intraductal carcinoma of the prostate].Pathologe. 2016 Feb;37(1):27-32. doi: 10.1007/s00292-015-0138-4. Pathologe. 2016. PMID: 26782033 Review. German.
-
Exposure to maternal obesogenic diet worsens some but not all pre-cancer phenotypes in a murine genetic model of prostate cancer.PLoS One. 2017 May 10;12(5):e0175764. doi: 10.1371/journal.pone.0175764. eCollection 2017. PLoS One. 2017. PMID: 28489892 Free PMC article.
References
-
- McNeal JE, Yemoto CE. Spread of adenocarcinoma within prostatic ducts and acini. Morphologic and clinical correlations. Am J Surg Pathol. 1996;20:802–814. - PubMed
-
- Cohen RJ, McNeal JE, Baillie T. Patterns of differentiation and proliferation in intraductal carcinoma of the prostate: significance for cancer progression. Prostate. 2000;43:11–19. - PubMed
-
- Wilcox G, Soh S, Chakraborty S, Scardino PT, Wheeler TM. Patterns of high-grade prostatic intraepithelial neoplasia associated with clinically aggressive prostate cancer. Hum Pathol. 1998;29:1119–1123. - PubMed
-
- Robinson BD, Epstein JI. Intraductal carcinoma of the prostate without invasive carcinoma on needle biopsy: emphasis on radical prostatectomy findings. J Urol. 2010;184:1328–1333. - PubMed
-
- Han B, Suleman K, Wang L, et al. ETS gene aberrations in atypical cribriform lesions of the prostate: implications for the distinction between intraductal carcinoma of the prostate and cribriform high-grade prostatic intraepithelial neoplasia. Am J Surg Pathol. 2010;34:478–485. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

