Periplasmic flagellar export apparatus protein, FliH, is involved in post-transcriptional regulation of FlaB, motility and virulence of the relapsing fever spirochete Borrelia hermsii
- PMID: 24009690
- PMCID: PMC3757020
- DOI: 10.1371/journal.pone.0072550
Periplasmic flagellar export apparatus protein, FliH, is involved in post-transcriptional regulation of FlaB, motility and virulence of the relapsing fever spirochete Borrelia hermsii
Abstract
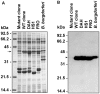
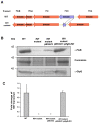
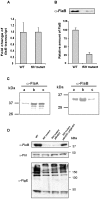
Spirochetes are bacteria characterized in part by rotating periplasmic flagella that impart their helical or flat-wave morphology and motility. While most other bacteria rely on a transcriptional cascade to regulate the expression of motility genes, spirochetes employ post-transcriptional mechanism(s) that are only partially known. In the present study, we characterize a spontaneous non-motile mutant of the relapsing fever spirochete Borrelia hermsii that was straight, non-motile and deficient in periplasmic flagella. We used next generation DNA sequencing of the mutant's genome, which when compared to the wild-type genome identified a 142 bp deletion in the chromosomal gene encoding the flagellar export apparatus protein FliH. Immunoblot and transcription analyses showed that the mutant phenotype was linked to the posttranscriptional deficiency in the synthesis of the major periplasmic flagellar filament core protein FlaB. Despite the lack of FlaB, the amount of FlaA produced by the fliH mutant was similar to the wild-type level. The turnover of the residual pool of FlaB produced by the fliH mutant was comparable to the wild-type spirochete. The non-motile mutant was not infectious in mice and its inoculation did not induce an antibody response. Trans-complementation of the mutant with an intact fliH gene restored the synthesis of FlaB, a normal morphology, motility and infectivity in mice. Therefore, we propose that the flagellar export apparatus protein regulates motility of B. hermsii at the post-transcriptional level by influencing the synthesis of FlaB.
Conflict of interest statement
Figures





Similar articles
-
The decrease in FlaA observed in a flaB mutant of Borrelia burgdorferi occurs posttranscriptionally.J Bacteriol. 2004 Jun;186(12):3703-11. doi: 10.1128/JB.186.12.3703-3711.2004. J Bacteriol. 2004. PMID: 15175283 Free PMC article.
-
Mutations in the Borrelia burgdorferi Flagellar Type III Secretion System Genes fliH and fliI Profoundly Affect Spirochete Flagellar Assembly, Morphology, Motility, Structure, and Cell Division.mBio. 2015 May 12;6(3):e00579-15. doi: 10.1128/mBio.00579-15. mBio. 2015. PMID: 25968649 Free PMC article.
-
Borrelia burgdorferi uniquely regulates its motility genes and has an intricate flagellar hook-basal body structure.J Bacteriol. 2008 Mar;190(6):1912-21. doi: 10.1128/JB.01421-07. Epub 2008 Jan 11. J Bacteriol. 2008. PMID: 18192386 Free PMC article.
-
The Flagellin-Specific Chaperone FliS of Borrelia burgdorferi Controls the Cytoplasmic Pool of Flagellins at the Level of Translation Initiation, Secretion, and Proteolysis.Mol Microbiol. 2025 Aug;124(2):173-187. doi: 10.1111/mmi.15380. Epub 2025 Jun 9. Mol Microbiol. 2025. PMID: 40491023 Free PMC article.
-
Spirochete periplasmic flagella and motility.J Mol Microbiol Biotechnol. 2000 Oct;2(4):345-54. J Mol Microbiol Biotechnol. 2000. PMID: 11075905 Review.
Cited by
-
Novel pegylated silver coated carbon nanotubes kill Salmonella but they are non-toxic to eukaryotic cells.J Nanobiotechnology. 2015 Mar 22;13:23. doi: 10.1186/s12951-015-0085-5. J Nanobiotechnology. 2015. PMID: 25888864 Free PMC article.
-
Role of Dual Specificity Phosphatase 1 (DUSP1) in influencing inflammatory pathways in macrophages modulated by Borrelia burgdorferi lipoproteins.bioRxiv [Preprint]. 2024 Nov 21:2024.11.20.624562. doi: 10.1101/2024.11.20.624562. bioRxiv. 2024. PMID: 39605372 Free PMC article. Preprint.
-
Motor rotation is essential for the formation of the periplasmic flagellar ribbon, cellular morphology, and Borrelia burgdorferi persistence within Ixodes scapularis tick and murine hosts.Infect Immun. 2015 May;83(5):1765-77. doi: 10.1128/IAI.03097-14. Epub 2015 Feb 17. Infect Immun. 2015. PMID: 25690096 Free PMC article.
-
Kinetics of tick infection by the relapsing fever spirochete Borrelia hermsii acquired through artificial membrane feeding chambers.Sci Rep. 2022 Aug 5;12(1):13479. doi: 10.1038/s41598-022-17500-9. Sci Rep. 2022. PMID: 35931720 Free PMC article.
-
Spirochetal motility and chemotaxis in the natural enzootic cycle and development of Lyme disease.Curr Opin Microbiol. 2015 Dec;28:106-13. doi: 10.1016/j.mib.2015.09.006. Epub 2015 Nov 2. Curr Opin Microbiol. 2015. PMID: 26519910 Free PMC article. Review.
References
-
- Felsenfeld O, Decker WJ, Wohlheiter JA, Rafy A (1965) Studies in Borreliae. II. Some immunologic, biochemical and physical properties of the antigenic components of Borrelia turicatae . J Immunol 94: 805–817. - PubMed
-
- Davis GE. Species unity or plurality of the relapsing fever spirochetes. In: Moulton FR, editor; 1942; Washington, D.C. American Association for Advancement of Science. 41–47.
-
- Canale-Parola E (1978) Motility and chemotaxis of spirochetes. Annu Rev Microbiol 32: 69–99. - PubMed
-
- Gage KL, Gilmore RD, Karstens RH, Schwan TG (1992) Detection of Rickettsia rickettsii in saliva, hemolymph and triturated tissues of infected Dermacentor andersoni ticks by polymerase chain reaction. Mol Cell Probes 6: 333–341. - PubMed
-
- Charon NW, Goldstein SF (2002) Genetics of motility and chemotaxis of a fascinating group of bacteria: the spirochetes. Annu Rev Genet 36: 47–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

