Stable cell fate changes in marrow cells induced by lung-derived microvesicles
- PMID: 24009878
- PMCID: PMC3760634
- DOI: 10.3402/jev.v1i0.18163
Stable cell fate changes in marrow cells induced by lung-derived microvesicles
Abstract
Background: Interest has been generated in the capacity of cellular-derived microvesicles to alter the fate of different target cells. Lung, liver, heart and brain-derived vesicles can alter the genetic phenotype of murine marrow cells; however, the stability of such changes and the mechanism of these changes remain unclear. In the present work, we show that lung-derived microvesicles (LDMV) alter the transcriptome and proteome of target marrow cells initially by mRNA and regulator(s) of transcription transfer, but that long term phenotype change is due solely to transfer of a transcriptional regulator with target cell.
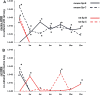
In vivo studies: Whole bone marrow cells (WBM) were co-cultured with LDMV (both isolated from male C57BL/6 mice) or cultured alone (control). One week later, cultured WBM was transplanted into lethally-irradiated female C57BL/6 mice. Recipient mice were sacrificed 6 weeks later and WBM, spleens and livers were examined for the presence of lung-specific gene expression, including surfactants A, B, C and D, aquaporin-5, and clara cell specific protein, via real-time RT-PCR. Immunohistochemistry was also performed on lungs to determine the number of transplanted marrow-derived (Y chromosome+) type II pneumocytes (prosurfactant C+). Mice transplanted with LDMV co-cultured WBM expressed pulmonary epithelial cell genes in the cells of their bone marrow, livers and spleens and over fivefold more transplanted marrow-derived Y+/prosurfactant C+cells could be found in their lungs (vs. control mice).
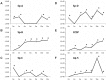
In vitro studies: WBM (from mice or rats) was cultured with or without LDMV (from mice or rats) for 1 week then washed and cultured alone. WBM was harvested at 2-week intervals for real-time RT-PCR analysis, using species-specific surfactant primers, and for Western Blot analysis. Proteomic and microRNA microarray analyses were also performed on cells. LDMV co-cultured WBM maintained expression of pulmonary epithelial cell genes and proteins for up to 12 weeks in culture. Surfactant produced at later time points was specific only to the species of the marrow cell in culture indicating de novo mRNA transcription. These findings, in addition to the altered protein and microRNA profiles of LDMV co-cultured WBM, support a stable transcriptional mechanism for these changes.
Conclusions: These data indicate that microvesicle alteration of cell fate is robust and long-term and represents an important new aspect of cellular biology.
Keywords: bone marrow cells; bone marrow transplant; lung; microvesicles; transcription factor.
Figures






References
-
- Aliotta JM, Passero M, Meharg J, Klinger J, Dooner MS, Pimentel J, et al. Stem cells and pulmonary metamorphosis: new concepts in repair and regeneration. J Cell Physiol. 2005;204:725–41. - PubMed
-
- Krause DS. Engraftment of bone marrow-derived epithelial cells. Ann N Y Acad Sci. 2006;1044:117–24. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

