Diverse subpopulations of vesicles secreted by different intracellular mechanisms are present in exosome preparations obtained by differential ultracentrifugation
- PMID: 24009879
- PMCID: PMC3760636
- DOI: 10.3402/jev.v1i0.18397
Diverse subpopulations of vesicles secreted by different intracellular mechanisms are present in exosome preparations obtained by differential ultracentrifugation
Abstract
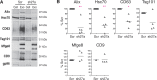
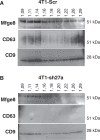
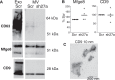
Exosomes are extracellular vesicles of 50 to 100 nm in diameter, released by many cell types. Exosomes are formed inside the cell in intracellular endosomal compartments and are secreted upon fusion of these compartments with the plasma membrane. Cells also secrete other types of membrane vesicles, for instance, by outward budding from the plasma membrane, and although some of them clearly differ from exosomes by their structural features (larger size), others are possibly more difficult to separate. Here, using Rab27a inhibition to modulate exosome secretion, we show the existence of at least 2 distinct populations of vesicles after purification by classical ultracentrifugation from mouse tumor cell conditioned medium. Rab27a inhibition lead to decreased vesicular secretion of some conventional markers of exosomes (CD63, Tsg101, Alix and Hsc70) but did not affect secretion of others (CD9 and Mfge8). By electron microscopy, CD9 was observed on vesicles of various sizes, ranging from 30 nm to more than 150 nm in diameter. Flotation onto sucrose gradients showed different proportions of CD63, CD9 and Mfge8 not only in fractions of densities classically described for exosomes (around 1.15 g/ml) but also in fractions of densities over 1.20 g/ml, indicating the presence of heterogenous vesicle populations. CD9 and Mfge8 were also found in large vesicles pelleted at low speed and can thus not be considered as specific components of endosome-derived vesicles. We propose that the most commonly used protocols for exosome preparations co-purify vesicles from endosomal and other origins, possibly the plasma membrane. Future work will be required to improve techniques for accurate purification and characterization of the different populations of extracellular vesicles.
Keywords: Rab proteins; exosomes; extracellular vesicles; markers; secretion machinery.
Figures



References
-
- Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–93. - PubMed
-
- Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19:43–51. - PubMed
-
- Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010;78:838–48. - PubMed
-
- George JN, Thoi LL, McManus LM, Reimann TA. Isolation of human platelet membrane microparticles from plasma and serum. Blood. 1982;60:834–40. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

