Extracellular Vesicle (EV) Array: microarray capturing of exosomes and other extracellular vesicles for multiplexed phenotyping
- PMID: 24009888
- PMCID: PMC3760630
- DOI: 10.3402/jev.v2i0.20920
Extracellular Vesicle (EV) Array: microarray capturing of exosomes and other extracellular vesicles for multiplexed phenotyping
Abstract
Background: Exosomes are one of the several types of cell-derived vesicles with a diameter of 30-100 nm. These extracellular vesicles are recognized as potential markers of human diseases such as cancer. However, their use in diagnostic tests requires an objective and high-throughput method to define their phenotype and determine their concentration in biological fluids. To identify circulating as well as cell culture-derived vesicles, the current standard is immunoblotting or a flow cytometrical analysis for specific proteins, both of which requires large amounts of purified vesicles.
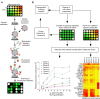
Methods: Based on the technology of protein microarray, we hereby present a highly sensitive Extracellular Vesicle (EV) Array capable of detecting and phenotyping exosomes and other extracellular vesicles from unpurified starting material in a high-throughput manner. To only detect the exosomes captured on the EV Array, a cocktail of antibodies against the tetraspanins CD9, CD63 and CD81 was used. These antibodies were selected to ensure that all exosomes captured are detected, and concomitantly excluding the detection of other types of microvesicles.
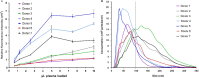
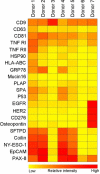
Results: The limit of detection (LOD) was determined on exosomes derived from the colon cancer cell line LS180. It clarified that supernatant from only approximately 10(4) cells was needed to obtain signals or that only 2.5×10(4) exosomes were required for each microarray spot (~1 nL). Phenotyping was performed on plasma (1-10 µL) from 7 healthy donors, which were applied to the EV Array with a panel of antibodies against 21 different cellular surface antigens and cancer antigens. For each donor, there was considerable heterogeneity in the expression levels of individual markers. The protein profiles of the exosomes (defined as positive for CD9, CD63 and CD81) revealed that only the expression level of CD9 and CD81 was approximately equal in the 7 donors. This implies questioning the use of CD63 as a standard exosomal marker since the expression level of this tetraspanin was considerably lower.
Keywords: EV Array; antigenic capturing; exosomes; extracellular vesicles; nanoparticle tracking analysis; phenotyping; plasma; protein microarray.
Figures

Similar articles
-
Nanoscale flow cytometry to distinguish subpopulations of prostate extracellular vesicles in patient plasma.Prostate. 2019 May;79(6):592-603. doi: 10.1002/pros.23764. Epub 2019 Jan 24. Prostate. 2019. PMID: 30680751
-
Exosomal proteins as potential diagnostic markers in advanced non-small cell lung carcinoma.J Extracell Vesicles. 2015 Mar 2;4:26659. doi: 10.3402/jev.v4.26659. eCollection 2015. J Extracell Vesicles. 2015. PMID: 25735706 Free PMC article.
-
Multiplexed Phenotyping of Small Extracellular Vesicles Using Protein Microarray (EV Array).Methods Mol Biol. 2017;1545:117-127. doi: 10.1007/978-1-4939-6728-5_8. Methods Mol Biol. 2017. PMID: 27943210
-
Plasma neuronal exosomes serve as biomarkers of cognitive impairment in HIV infection and Alzheimer's disease.J Neurovirol. 2019 Oct;25(5):702-709. doi: 10.1007/s13365-018-0695-4. Epub 2019 Jan 4. J Neurovirol. 2019. PMID: 30610738 Free PMC article. Review.
-
Extracellular Vesicles: Recent Developments in Technology and Perspectives for Cancer Liquid Biopsy.Recent Results Cancer Res. 2020;215:319-344. doi: 10.1007/978-3-030-26439-0_17. Recent Results Cancer Res. 2020. PMID: 31605237 Review.
Cited by
-
Methods for extracellular vesicles isolation in a hospital setting.Front Immunol. 2015 Feb 13;6:50. doi: 10.3389/fimmu.2015.00050. eCollection 2015. Front Immunol. 2015. PMID: 25762995 Free PMC article.
-
New Approaches and Biomarker Candidates for the Early Detection of Ovarian Cancer.Front Bioeng Biotechnol. 2022 Feb 10;10:819183. doi: 10.3389/fbioe.2022.819183. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35223789 Free PMC article. No abstract available.
-
Amphipathic helical peptide-based fluorogenic probes for a marker-free analysis of exosomes based on membrane-curvature sensing.RSC Adv. 2020 Oct 19;10(63):38323-38327. doi: 10.1039/d0ra07763a. eCollection 2020 Oct 15. RSC Adv. 2020. PMID: 35517518 Free PMC article.
-
Platelets, endothelial cells and leukocytes contribute to the exercise-triggered release of extracellular vesicles into the circulation.J Extracell Vesicles. 2019 May 28;8(1):1615820. doi: 10.1080/20013078.2019.1615820. eCollection 2019. J Extracell Vesicles. 2019. PMID: 31191831 Free PMC article.
-
Membrane-binding peptides for extracellular vesicles on-chip analysis.J Extracell Vesicles. 2020 Apr 17;9(1):1751428. doi: 10.1080/20013078.2020.1751428. eCollection 2020. J Extracell Vesicles. 2020. PMID: 32363015 Free PMC article.
References
-
- Lee TH, D'Asti E, Magnus N, Al-Nedawi K, Meehan B, Rak J. Microvesicles as mediators of intercellular communication in cancer – the emerging science of cellular “debris”. Semin Immunopathol. 2011;33:455–67. - PubMed
-
- Simons M, Raposo G. Exosomes-vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21:575–81. - PubMed
-
- Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nature reviews. Immunology. 2009;9:581–93. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

