An insight into the emerging role of regional medical advisor in the pharmaceutical industry
- PMID: 24010061
- PMCID: PMC3757584
- DOI: 10.4103/2229-3485.115386
An insight into the emerging role of regional medical advisor in the pharmaceutical industry
Abstract
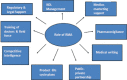
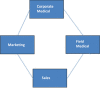
The position of regional medical advisor (RMA) is relatively new in the pharmaceutical industry and its roles and responsibility are still evolving. The RMA is a field based position whose main mission is to foster collaborative relationships with the key opinion leaders (KOLs) and to facilitate the exchange of unbiased scientific information between the medical community and the company. Field-based medical liaison teams are expanding world-wide as part of the pharmaceutical industry's increased focus on global operations including emerging markets. Now, the position of the RMA has evolved into comprehensive, complex, highly interactive, targeted, highly strategic, innovative, and independent role since its inception by the Upjohn Company in 1967. The major objective of the RMA is to develop the professional relationships with the health-care community, particularly KOLs, through peer-to-peer contact. The RMA can facilitate investigator-initiated clinical research proposals from approval until completion, presentation, and publication. It is possible for a RMA to have valuable access to KOLs through his expertise in the clinical research. The RMA can assist in the development, review, and follow-up of the clinical studies initiated within the relevant therapeutic area at the regional/local level. The RMA can lead regional/local clinical projects to ensure that all clinical trials are conducted in compliance with the International Conference of Harmonisation Good Clinical Practice (ICH GCP) guidelines.
Keywords: Clinical research; key opinion leaders; medical science liaison; medico-marketing; pharmaceutical industry; regional medical advisor.
Conflict of interest statement
Figures
References
-
- British Medical Association. The pharmaceutical physician. 2007. [Last accessed on 2012 Sep 16]. Available from: http://www.careers.cam.ac.uk/sectors/health/files/Pharmaceutical_Physici... .
-
- Albert E, Sass C. 2nd ed. Author House; 2007. The Medical Science Liaison: An A to Z Guide.
-
- Logan G. Medical Science Liaison (MSL). Reprint from PIPELINE issue 34. Takeda UK Ltd Write-up by Philippa Shiels, AXESS Ltd; 2011. [Last accessed on 2012 Sep 20]. Understanding, engaging and interacting. Available from: http://www.axess.co.uk/wp-content/uploads/2011/09/MSL-article-1.pdf .
-
- Morgan KD, Domann DE, Collins GE, Massey KL, Moss RJ. History and evolution of field-based medical programs. Drug Inf J. 2000;34:1049–52.
-
- Shearer DM. Medical Science Liasion: Emerging Roles. Integrated Clinical Trial Services White Paper on Medical Science Liaisons (MSL) 2009. [Last accessed on 2012 Sep 27]. Available from: http://www.integratedtrials.com/news.htm .
LinkOut - more resources
Full Text Sources
Other Literature Sources