Novel automated tracking analysis of particles subjected to shear flow: kindlin-3 role in B cells
- PMID: 24010654
- PMCID: PMC3762340
- DOI: 10.1016/j.bpj.2013.06.051
Novel automated tracking analysis of particles subjected to shear flow: kindlin-3 role in B cells
Abstract
Shear flow assays are used to mimic the influence of physiological shear force in diverse situations such as leukocyte rolling and arrest on the vasculature, capture of nanoparticles, and bacterial adhesion. Analysis of such assays usually involves manual counting, is labor-intensive, and is subject to bias. We have developed the Leukotrack program that incorporates a novel (to our knowledge) segmentation routine capable of reliable detection of cells in phase contrast images. The program also automatically tracks rolling cells in addition to those that are more firmly attached and migrating in random directions. We demonstrate its use in the analysis of lymphocyte arrest mediated by one or more active conformations of the integrin LFA-1. Activation of LFA-1 is a multistep process that depends on several proteins including kindlin-3, the protein that is mutated in leukocyte adhesion deficiency-III patients. We find that the very first stage of LFA-1-mediated attaching is unable to proceed in the absence of kindlin-3. Our evidence indicates that kindlin-3-mediated high-affinity LFA-1 controls both the early transient integrin-dependent adhesions in addition to the final stable adhesions made under flow conditions.
Copyright © 2013 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
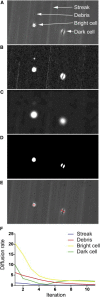
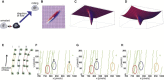
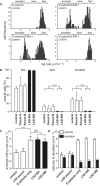
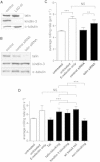
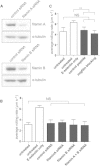
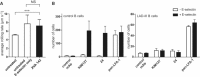
Comment in
-
Clocking leukocytes reveal dynamics of integrin braking.Biophys J. 2013 Sep 3;105(5):1091-2. doi: 10.1016/j.bpj.2013.07.030. Biophys J. 2013. PMID: 24010651 Free PMC article. No abstract available.
Similar articles
-
Loss of Kindlin-3 in LAD-III eliminates LFA-1 but not VLA-4 adhesiveness developed under shear flow conditions.Blood. 2009 Sep 10;114(11):2344-53. doi: 10.1182/blood-2009-04-218636. Epub 2009 Jul 17. Blood. 2009. PMID: 19617577
-
The kindlin 3 pleckstrin homology domain has an essential role in lymphocyte function-associated antigen 1 (LFA-1) integrin-mediated B cell adhesion and migration.J Biol Chem. 2013 May 24;288(21):14852-62. doi: 10.1074/jbc.M112.434621. Epub 2013 Apr 17. J Biol Chem. 2013. PMID: 23595985 Free PMC article.
-
Distinct roles for talin-1 and kindlin-3 in LFA-1 extension and affinity regulation.Blood. 2012 May 3;119(18):4275-82. doi: 10.1182/blood-2011-08-373118. Epub 2012 Mar 19. Blood. 2012. PMID: 22431571 Free PMC article.
-
Leukocyte arrest: Biomechanics and molecular mechanisms of β2 integrin activation.Biorheology. 2015;52(5-6):353-77. doi: 10.3233/BIR-15085. Biorheology. 2015. PMID: 26684674 Free PMC article. Review.
-
Kindlin: helper, co-activator, or booster of talin in integrin activation?Curr Opin Hematol. 2011 Sep;18(5):356-60. doi: 10.1097/MOH.0b013e3283497f09. Curr Opin Hematol. 2011. PMID: 21730832 Review.
Cited by
-
Clocking leukocytes reveal dynamics of integrin braking.Biophys J. 2013 Sep 3;105(5):1091-2. doi: 10.1016/j.bpj.2013.07.030. Biophys J. 2013. PMID: 24010651 Free PMC article. No abstract available.
-
Manual and Automatic Image Analysis Segmentation Methods for Blood Flow Studies in Microchannels.Micromachines (Basel). 2021 Mar 18;12(3):317. doi: 10.3390/mi12030317. Micromachines (Basel). 2021. PMID: 33803615 Free PMC article. Review.
-
Kindlin-3 in the immune system.Am J Clin Exp Immunol. 2014 Feb 27;3(1):37-42. eCollection 2014. Am J Clin Exp Immunol. 2014. PMID: 24660120 Free PMC article. Review.
References
-
- Nourshargh S., Hordijk P.L., Sixt M. Breaching multiple barriers: leukocyte motility through venular walls and the interstitium. Nat. Rev. Mol. Cell Biol. 2010;11:366–378. - PubMed
-
- Chaffer C.L., Weinberg R.A. A perspective on cancer cell metastasis. Science. 2011;331:1559–1564. - PubMed
-
- Kim M.J., Rhee K. Computational analysis of nanoparticle adhesion to endothelium: effects of kinetic rate constants and wall shear rates. Med. Biol. Eng. Comput. 2011;49:733–741. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

