Inactivated influenza virus vaccine is efficient and reduces IL-4 and IL-6 in allergic asthma mice
- PMID: 24010941
- PMCID: PMC4634242
- DOI: 10.1111/irv.12150
Inactivated influenza virus vaccine is efficient and reduces IL-4 and IL-6 in allergic asthma mice
Abstract
Background: Allergic asthma is a globally respiratory inflammatory disease. Influenza virus is a respiratory pathogen that causes yearly epidemics and results in high rates of morbidity and mortality. Patients with allergic asthma had a more severe symptom and a higher mortality when they were infected with influenza virus. Hence, influenza vaccination is recommended for patients with asthma.
Objectives: We evaluated the efficacy and effects of influenza vaccination on allergic asthma in a mouse model.
Methods: Ovalbumin-immunized mice were inoculated with inactivated influenza virus A/Puerto Rico/8/34 (PR8) as vaccines and morbidity or mortality and allergic asthma features of these mice were analyzed.
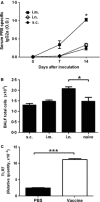
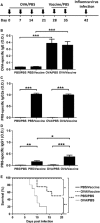
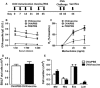
Results: Mice inoculated with inactivated PR8 induced high levels of anti-PR8 IgG2a and upregulation of Toll-like receptor (TLR) 7. Vaccinated allergic mice were healthy when they were challenged with live influenza virus while none of non-vaccinated allergic mice survived. Furthermore, inactivated influenza virus vaccine induced neither extra airway inflammation nor asthma features such as IgE, airway hyper-reactivity, and eosinophilia in allergic mice. Particularly, decreased frequency of immune cell infiltrated airways and Th2 cytokines IL-4 and IL-6 production in the bronchoalveolar lavage fluid were noted in vaccinated allergic mice. These results suggested that inactivated influenza virus vaccine is efficient to protect allergic mice from further influenza infection, and it does not exacerbate but reduces IL-4 and IL-6 of allergic asthma.
Conclusion: Influenza vaccination is essential and efficient for allergic subjects to protect influenza virus infection.
Keywords: IL-4; IL-6; PR8; asthma; vaccine.
© 2013 John Wiley & Sons Ltd.
Figures


References
-
- Holgate ST, Polosa R. Treatment strategies for allergy and asthma. Nat Rev Immunol 2008; 8:218–230. - PubMed
-
- Chuang YH, Yang YH, Wu SJ, Chiang BL. Gene therapy for allergic diseases. Curr Gene Ther 2009; 9:185–191. - PubMed
-
- Ong BA, Forester J, Fallot A. Does influenza vaccination improve pediatric asthma outcomes? J Asthma 2009; 46:477–480. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

