Nonthermal ablation with microbubble-enhanced focused ultrasound close to the optic tract without affecting nerve function
- PMID: 24010975
- PMCID: PMC4028139
- DOI: 10.3171/2013.8.JNS122387
Nonthermal ablation with microbubble-enhanced focused ultrasound close to the optic tract without affecting nerve function
Abstract
Object: Tumors at the skull base are challenging for both resection and radiosurgery given the presence of critical adjacent structures, such as cranial nerves, blood vessels, and brainstem. Magnetic resonance imaging-guided thermal ablation via laser or other methods has been evaluated as a minimally invasive alternative to these techniques in the brain. Focused ultrasound (FUS) offers a noninvasive method of thermal ablation; however, skull heating limits currently available technology to ablation at regions distant from the skull bone. Here, the authors evaluated a method that circumvents this problem by combining the FUS exposures with injected microbubble-based ultrasound contrast agent. These microbubbles concentrate the ultrasound-induced effects on the vasculature, enabling an ablation method that does not cause significant heating of the brain or skull.
Methods: In 29 rats, a 525-kHz FUS transducer was used to ablate tissue structures at the skull base that were centered on or adjacent to the optic tract or chiasm. Low-intensity, low-duty-cycle ultrasound exposures (sonications) were applied for 5 minutes after intravenous injection of an ultrasound contrast agent (Definity, Lantheus Medical Imaging Inc.). Using histological analysis and visual evoked potential (VEP) measurements, the authors determined whether structural or functional damage was induced in the optic tract or chiasm.
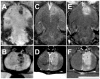
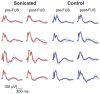
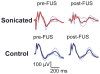
Results: Overall, while the sonications produced a well-defined lesion in the gray matter targets, the adjacent tract and chiasm had comparatively little or no damage. No significant changes (p > 0.05) were found in the magnitude or latency of the VEP recordings, either immediately after sonication or at later times up to 4 weeks after sonication, and no delayed effects were evident in the histological features of the optic nerve and retina.
Conclusions: This technique, which selectively targets the intravascular microbubbles, appears to be a promising method of noninvasively producing sharply demarcated lesions in deep brain structures while preserving function in adjacent nerves. Because of low vascularity--and thus a low microbubble concentration--some large white matter tracts appear to have some natural resistance to this type of ablation compared with gray matter. While future work is needed to develop methods of monitoring the procedure and establishing its safety at deep brain targets, the technique does appear to be a potential solution that allows FUS ablation of deep brain targets while sparing adjacent nerve structures.
Figures








References
-
- Aubry JF, Tanter M, Pernot M, Thomas JL, Fink M. Experimental demonstration of noninvasive transskull adaptive focusing based on prior computed tomography scans. J Acoust Soc Am. 2003;113:84–93. - PubMed
-
- Ballantine HT, Jr, Bell E, Manlapaz J. Progress and problems in the neurological applications of focused ultrasound. J Neurosurg. 1960;17:858–876. - PubMed
-
- Bamber JC. Ultrasonic attenuation in fresh human tissues. Ultrasonics. 1981;19:187–188. - PubMed
-
- Billard BE, Hynynen K, Roemer RB. Effects of physical parameters on high temperature ultrasound hyperthermia. Ultrasound Med Biol. 1990;16:409–420. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

