The Toxoplasma gondii calcium-dependent protein kinase 7 is involved in early steps of parasite division and is crucial for parasite survival
- PMID: 24011186
- PMCID: PMC4091637
- DOI: 10.1111/cmi.12186
The Toxoplasma gondii calcium-dependent protein kinase 7 is involved in early steps of parasite division and is crucial for parasite survival
Abstract
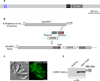
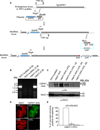
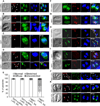
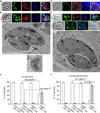
Apicomplexan parasites express various calcium-dependent protein kinases (CDPKs), and some of them play essential roles in invasion and egress. Five of the six CDPKs conserved in most Apicomplexa have been studied at the molecular and cellular levels in Plasmodium species and/or in Toxoplasma gondii parasites, but the function of CDPK7 was so far uncharacterized. In T. gondii, during intracellular replication, two parasites are formed within a mother cell through a unique process called endodyogeny. Here we demonstrate that the knock-down of CDPK7 protein in T. gondii results in pronounced defects in parasite division and a major growth deficiency, while it is dispensable for motility, egress and microneme exocytosis. In cdpk7-depleted parasites, the overall DNA content was not impaired, but the polarity of daughter cells budding and the fate of several subcellular structures or proteins involved in cell division were affected, such as the centrosomes and the kinetochore. Overall, our data suggest that CDPK7 is crucial for proper maintenance of centrosome integrity required for the initiation of endodyogeny. Our findings provide a first insight into the probable role of calcium-dependent signalling in parasite multiplication, in addition to its more widely explored role in invasion and egress.
© 2013 John Wiley & Sons Ltd.
Figures




Similar articles
-
The conserved apicomplexan Aurora kinase TgArk3 is involved in endodyogeny, duplication rate and parasite virulence.Cell Microbiol. 2016 Aug;18(8):1106-1120. doi: 10.1111/cmi.12571. Epub 2016 Feb 22. Cell Microbiol. 2016. PMID: 26833682 Free PMC article.
-
Loss of the Conserved Alveolate Kinase MAPK2 Decouples Toxoplasma Cell Growth from Cell Division.mBio. 2020 Nov 10;11(6):e02517-20. doi: 10.1128/mBio.02517-20. mBio. 2020. PMID: 33173004 Free PMC article.
-
The essential kinase TgGSK regulates centrosome segregation and endodyogeny in Toxoplasma gondii.mSphere. 2025 Apr 29;10(4):e0011125. doi: 10.1128/msphere.00111-25. Epub 2025 Mar 28. mSphere. 2025. PMID: 40152591 Free PMC article.
-
Toxoplasma and Plasmodium protein kinases: roles in invasion and host cell remodelling.Int J Parasitol. 2012 Jan;42(1):21-32. doi: 10.1016/j.ijpara.2011.11.007. Epub 2011 Dec 4. Int J Parasitol. 2012. PMID: 22154850 Free PMC article. Review.
-
The calcium signaling toolkit of the Apicomplexan parasites Toxoplasma gondii and Plasmodium spp.Cell Calcium. 2015 Mar;57(3):186-93. doi: 10.1016/j.ceca.2014.12.010. Epub 2014 Dec 31. Cell Calcium. 2015. PMID: 25605521 Free PMC article. Review.
Cited by
-
Toxoplasma gondii's Basal Complex: The Other Apicomplexan Business End Is Multifunctional.Front Cell Infect Microbiol. 2022 Apr 29;12:882166. doi: 10.3389/fcimb.2022.882166. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35573773 Free PMC article.
-
TgCep250 is dynamically processed through the division cycle and is essential for structural integrity of the Toxoplasma centrosome.Mol Biol Cell. 2019 May 1;30(10):1160-1169. doi: 10.1091/mbc.E18-10-0608. Epub 2019 Mar 13. Mol Biol Cell. 2019. PMID: 30865554 Free PMC article.
-
Calcium signaling and the lytic cycle of the Apicomplexan parasite Toxoplasma gondii.Biochim Biophys Acta Mol Cell Res. 2018 Nov;1865(11 Pt B):1846-1856. doi: 10.1016/j.bbamcr.2018.08.004. Epub 2018 Aug 10. Biochim Biophys Acta Mol Cell Res. 2018. PMID: 30992126 Free PMC article. Review.
-
The FIKK kinase of Toxoplasma gondii is not essential for the parasite's lytic cycle.Int J Parasitol. 2016 May;46(5-6):323-32. doi: 10.1016/j.ijpara.2016.01.001. Epub 2016 Feb 6. Int J Parasitol. 2016. PMID: 26859096 Free PMC article.
-
Exploring protein myristoylation in Toxoplasma gondii.Exp Parasitol. 2019 Aug;203:8-18. doi: 10.1016/j.exppara.2019.05.007. Epub 2019 May 28. Exp Parasitol. 2019. PMID: 31150653 Free PMC article.
References
-
- Achbarou A, Mercereau-Puijalon O, Autheman JM, Fortier B, Camus D, Dubremetz JF. Characterization of microneme proteins of Toxoplasma gondii. Mol Biochem Parasitol. 1991;47:223–233. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

