Diffuse intrinsic pontine glioma treated with prolonged temozolomide and radiotherapy--results of a United Kingdom phase II trial (CNS 2007 04)
- PMID: 24011536
- PMCID: PMC3853623
- DOI: 10.1016/j.ejca.2013.08.006
Diffuse intrinsic pontine glioma treated with prolonged temozolomide and radiotherapy--results of a United Kingdom phase II trial (CNS 2007 04)
Abstract
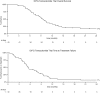
Diffuse intrinsic pontine glioma (DIPG) has a dismal prognosis with no chemotherapy regimen so far resulting in any significant improvement over standard radiotherapy. In this trial, a prolonged regimen (21/28d) of temozolomide was studied with the aim of overcoming O(6)-methylguanine methyltransferase (MGMT) mediated resistance. Forty-three patients with a defined clinico-radiological diagnosis of DIPG received radiotherapy and concomitant temozolomide (75 mg/m(2)) after which up to 12 courses of 21d of adjuvant temozolomide (75-100mg/m(2)) were given 4 weekly. The trial used a 2-stage design and passed interim analysis. At diagnosis median age was 8 years (2-20 years), 81% had cranial nerve abnormalities, 76% ataxia and 57% long tract signs. Median Karnofsky/Lansky score was 80 (10-100). Patients received a median of three courses of adjuvant temozolomide, five received all 12 courses and seven did not start adjuvant treatment. Three patients were withdrawn from study treatment due to haematological toxicity and 10 had a dose reduction. No other significant toxicity related to temozolomide was noted. Overall survival (OS) (95% confidence interval (CI)) was 56% (40%, 69%) at 9 months, 35% (21%, 49%) at 1 year and 17% (7%, 30%) at 2 years. Median survival was 9.5 months (range 7.5-11.4 months). There were five 2-year survivors with a median age of 13.6 years at diagnosis. This trial demonstrated no survival benefit of the addition of dose dense temozolomide, to standard radiotherapy in children with classical DIPG. However, a subgroup of adolescent DIPG patients did have a prolonged survival, which needs further exploration.
Keywords: Diffuse intrinsic pontine glioma (DIPG); Temozolomide.
Copyright © 2013 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures
References
-
- Hargrave D., Bartels U., Bouffet E. Diffuse brainstem glioma in children: critical review of clinical trials. Lancet Oncol. 2006;7:241–248. - PubMed
-
- Jansen M.H., van Vuurden D.G., Vandertop W.P., Kaspers G.J. Diffuse intrinsic pontine gliomas: a systematic update on clinical trials and biology. Cancer Treat Rev. 2012;38:27–35. - PubMed
-
- Freeman C.R., Suissa S. Brain stem tumors in children: results of a survey of 62 patients treated with radiotherapy. Int J Radiat Oncol Biol Phys. 1986;12:1823–1828. - PubMed
-
- Freeman C.R., Krischer J.P., Sanford R.A. Final results of a study of escalating doses of hyperfractionated radiotherapy in brain stem tumors in children: a Pediatric Oncology Group study. Int J Radiat Oncol Biol Phys. 1993;27:197–206. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials