IL-17 family: cytokines, receptors and signaling
- PMID: 24011563
- PMCID: PMC3867811
- DOI: 10.1016/j.cyto.2013.07.022
IL-17 family: cytokines, receptors and signaling
Abstract
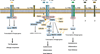
The interleukin 17 (IL-17) family, a subset of cytokines consisting of IL-17A-F, plays crucial roles in host defense against microbial organisms and in the development of inflammatory diseases. Although IL-17A is the signature cytokine produced by T helper 17 (Th17) cells, IL-17A and other IL-17 family cytokines have multiple sources ranging from immune cells to non-immune cells. The IL-17 family signals via their correspondent receptors and activates downstream pathways that include NFκB, MAPKs and C/EBPs to induce the expression of anti-microbial peptides, cytokines and chemokines. The proximal adaptor Act1 is a common mediator during the signaling of all IL-17 cytokines so far and is thus involved in IL-17 mediated host defense and IL-17-driven autoimmune conditions. This review will give an overview and recent updates on the IL-17 family, the activation and regulation of IL-17 signaling as well as diseases associated with this cytokine family.
Keywords: Act1; IL-17R; Interleukin 17; Signaling transduction.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors have no conflicting financial interests.
Figures
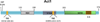
References
-
- Rouvier E, Luciani MF, Mattei MG, Denizot F, Golstein P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J Immunol. 1993;150(12):5445–5456. - PubMed
-
- Yao Z, Painter SL, Fanslow WC, Ulrich D, Macduff BM, Spriggs MK, et al. Human IL-17: a novel cytokine derived from T cells. J Immunol. 1995;155(12):5483–5486. - PubMed
-
- Starnes T, Broxmeyer HE, Robertson MJ, Hromas R. Cutting edge: IL-17D, a novel member of the IL-17 family, stimulates cytokine production and inhibits hemopoiesis. J Immunol. 2002;169(2):642–646. - PubMed
-
- Lee J, Ho WH, Maruoka M, Corpuz RT, Baldwin DT, Foster JS, et al. IL-17E, a novel proinflammatory ligand for the IL-17 receptor homolog IL-17Rh1. J Biol Chem. 2001;276(2):1660–1664. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

