c-Ski activates cancer-associated fibroblasts to regulate breast cancer cell invasion
- PMID: 24011664
- PMCID: PMC3864893
- DOI: 10.1016/j.molonc.2013.08.007
c-Ski activates cancer-associated fibroblasts to regulate breast cancer cell invasion
Abstract
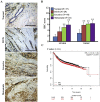
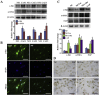
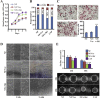
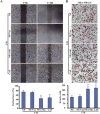
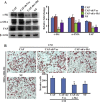
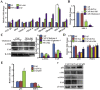
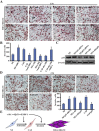
Aberrant expression of c-Ski oncoprotein in some tumor cells has been shown to be associated with cancer development. However, the role of c-Ski in cancer-associated fibroblasts (CAFs) of tumor microenvironment has not been characterized. In the current study, we found that c-Ski is highly expressed in CAFs derived from breast carcinoma microenvironment and this CAF-associated c-Ski expression is associated with invasion and metastasis of human breast tumors. We showed that c-Ski overexpression in immortalized breast normal fibroblasts (NFs) induces conversion to breast CAFs by repressing p53 and thereby upregulating SDF-1 in NFs. SDF-1 treatment or p53 knockdown in NFs had similar effects on the activation of NFs as c-Ski overexpression. The c-Ski-activated CAFs show increased proliferation, migration, invasion and contraction compared with NFs. Furthermore, c-Ski-activated CAFs facilitated the migration and invasion of MDA-MB-231 breast cancer cells. Our data suggest that c-Ski is an important regulator in the activation of CAFs and may serve as a potential therapeutic target to block breast cancer progression.
Keywords: CAFs; P53; SDF-1; Tumor microenvironment; c-Ski.
Copyright © 2013 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Overexpression of c-Ski promotes cell proliferation, invasion and migration of gastric cancer associated fibroblasts.Kaohsiung J Med Sci. 2019 Apr;35(4):214-221. doi: 10.1002/kjm2.12042. Epub 2019 Mar 21. Kaohsiung J Med Sci. 2019. PMID: 30896889 Free PMC article.
-
Stromal miR-200s contribute to breast cancer cell invasion through CAF activation and ECM remodeling.Cell Death Differ. 2016 Jan;23(1):132-45. doi: 10.1038/cdd.2015.78. Epub 2015 Jun 12. Cell Death Differ. 2016. PMID: 26068592 Free PMC article.
-
Breast cancer-derived exosomes regulate cell invasion and metastasis in breast cancer via miR-146a to activate cancer associated fibroblasts in tumor microenvironment.Exp Cell Res. 2020 Jun 15;391(2):111983. doi: 10.1016/j.yexcr.2020.111983. Epub 2020 Apr 5. Exp Cell Res. 2020. PMID: 32268136
-
The role of cancer-associated fibroblasts in breast cancer pathobiology.Histol Histopathol. 2016 Apr;31(4):371-8. doi: 10.14670/HH-11-700. Epub 2015 Dec 2. Histol Histopathol. 2016. PMID: 26627101 Review.
-
Crosstalk and plasticity driving between cancer-associated fibroblasts and tumor microenvironment: significance of breast cancer metastasis.J Transl Med. 2023 Nov 17;21(1):827. doi: 10.1186/s12967-023-04714-2. J Transl Med. 2023. PMID: 37978384 Free PMC article. Review.
Cited by
-
GPR30-mediated HMGB1 upregulation in CAFs induces autophagy and tamoxifen resistance in ERα-positive breast cancer cells.Aging (Albany NY). 2021 Jun 28;13(12):16178-16197. doi: 10.18632/aging.203145. Epub 2021 Jun 28. Aging (Albany NY). 2021. PMID: 34182538 Free PMC article.
-
Oxidized ATM-mediated glycolysis enhancement in breast cancer-associated fibroblasts contributes to tumor invasion through lactate as metabolic coupling.EBioMedicine. 2019 Mar;41:370-383. doi: 10.1016/j.ebiom.2019.02.025. Epub 2019 Feb 22. EBioMedicine. 2019. PMID: 30799198 Free PMC article.
-
HepaCAM inhibits cell proliferation and invasion in prostate cancer by suppressing nuclear translocation of the androgen receptor via its cytoplasmic domain.Mol Med Rep. 2019 Mar;19(3):2115-2124. doi: 10.3892/mmr.2019.9841. Epub 2019 Jan 10. Mol Med Rep. 2019. PMID: 30664187 Free PMC article.
-
The role of cancer-associated fibroblasts in breast cancer metastasis.Front Oncol. 2023 Jul 11;13:1194835. doi: 10.3389/fonc.2023.1194835. eCollection 2023. Front Oncol. 2023. PMID: 37496657 Free PMC article. Review.
-
Breast cancer-associated fibroblasts: their roles in tumor initiation, progression and clinical applications.Front Med. 2016 Mar;10(1):33-40. doi: 10.1007/s11684-016-0431-5. Epub 2016 Jan 20. Front Med. 2016. PMID: 26791754 Review.
References
-
- Ao, M. , Franco, O.E. , Park, D. , Raman, D. , Williams, K. , Hayward, S.W. , 2007. Cross-talk between paracrine-acting cytokine and chemokine pathways promotes malignancy in benign human prostatic epithelium. Cancer Res.. 67, 4244–4253. - PubMed
-
- Bonnon, C. , Atanasoski, S. , 2012. c-Ski in health and disease. Cell Tissue Res.. 347, 51–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

