Understanding of dopant-induced osteogenesis and angiogenesis in calcium phosphate ceramics
- PMID: 24012308
- PMCID: PMC3825404
- DOI: 10.1016/j.tibtech.2013.06.005
Understanding of dopant-induced osteogenesis and angiogenesis in calcium phosphate ceramics
Abstract
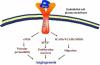
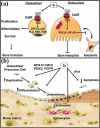
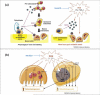
General trends in synthetic bone grafting materials are shifting towards approaches that can illicit osteoinductive properties. Pharmacologics and biologics have been used in combination with calcium phosphate (CaP) ceramics, however, they have recently become the target of scrutiny over safety. The importance of trace elements in natural bone health is well documented. Ions, for example, lithium, zinc, magnesium, manganese, silicon, strontium, etc., have been shown to increase osteogenesis and neovascularization. Incorporation of dopants (trace metal ions) into CaPs can provide a platform for safe and efficient delivery in clinical applications where increased bone healing is favorable. This review highlights the use of trace elements in CaP biomaterials, and offers an insight into the mechanisms of how metal ions can enhance both osteogenesis and angiogenesis.
Keywords: angiogenesis; bone remodeling; calcium phosphates; dopants or trace metal ions; osteogenesis.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures




References
-
- Carano RAD, Filvaroff EH. Angiogenesis and bone repair. Drug Discov Today. 2003;8:980–9. - PubMed
-
- Gerber HP, Ferrara N. Angiogenesis and bone growth. Trends Cardiovasc Med. 2000;10:223–8. - PubMed
-
- Geiger M. Collagen sponges for bone regeneration with rhBMP-2. Adv Drug Deliv Rev. 2003;55:1613–29. - PubMed
-
- Tamai N, Myoui A, Hirao M, et al. A new biotechnology for articular cartilage repair: subchondral implantation of a composite of interconnected porous hydroxyapatite, synthetic polymer (PLA-PEG), and bone morphogenetic protein-2 (rhBMP-2). Osteoarthritis Cartilage. 2005;13:405–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

