Spatial organization of Hippo signaling at the plasma membrane mediated by the tumor suppressor Merlin/NF2
- PMID: 24012335
- PMCID: PMC3835333
- DOI: 10.1016/j.cell.2013.08.025
Spatial organization of Hippo signaling at the plasma membrane mediated by the tumor suppressor Merlin/NF2
Abstract
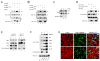
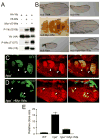
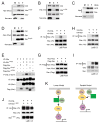
Although Merlin/NF2 was discovered two decades ago as a tumor suppressor underlying Neurofibromatosis type II, its precise molecular mechanism remains poorly understood. Recent studies in Drosophila revealed a potential link between Merlin and the Hippo pathway by placing Merlin genetically upstream of the kinase Hpo/Mst. In contrast to the commonly depicted linear model of Merlin functioning through Hpo/Mst, here we show that in both Drosophila and mammals, Merlin promotes downstream Hippo signaling without activating the intrinsic kinase activity of Hpo/Mst. Instead, Merlin directly binds and recruits the effector kinase Wts/Lats to the plasma membrane. Membrane recruitment, in turn, promotes Wts phosphorylation by the Hpo-Sav kinase complex. We further show that disruption of the actin cytoskeleton promotes Merlin-Wts interactions, which implicates Merlin in actin-mediated regulation of Hippo signaling. Our findings elucidate an important molecular function of Merlin and highlight the plasma membrane as a critical subcellular compartment for Hippo signal transduction.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures

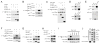

Similar articles
-
Kibra and Merlin Activate the Hippo Pathway Spatially Distinct from and Independent of Expanded.Dev Cell. 2017 Mar 13;40(5):478-490.e3. doi: 10.1016/j.devcel.2017.02.004. Dev Cell. 2017. PMID: 28292426 Free PMC article.
-
Phase separation of Hippo signalling complexes.EMBO J. 2023 Mar 15;42(6):e112863. doi: 10.15252/embj.2022112863. Epub 2023 Feb 20. EMBO J. 2023. PMID: 36807601 Free PMC article.
-
Identification of Happyhour/MAP4K as Alternative Hpo/Mst-like Kinases in the Hippo Kinase Cascade.Dev Cell. 2015 Sep 28;34(6):642-55. doi: 10.1016/j.devcel.2015.08.014. Epub 2015 Sep 10. Dev Cell. 2015. PMID: 26364751 Free PMC article.
-
[Research progress of Merlin protein and its signal pathway in tumor].Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2016 May 5;30(9):757-762. doi: 10.13201/j.issn.1001-1781.2016.09.023. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2016. PMID: 29771032 Review. Chinese.
-
NF2/Merlin Inactivation and Potential Therapeutic Targets in Mesothelioma.Int J Mol Sci. 2018 Mar 26;19(4):988. doi: 10.3390/ijms19040988. Int J Mol Sci. 2018. PMID: 29587439 Free PMC article. Review.
Cited by
-
Pathomechanisms in schwannoma development and progression.Oncogene. 2020 Aug;39(32):5421-5429. doi: 10.1038/s41388-020-1374-5. Epub 2020 Jul 2. Oncogene. 2020. PMID: 32616891 Free PMC article. Review.
-
The Hippo signalling pathway and its implications in human health and diseases.Signal Transduct Target Ther. 2022 Nov 8;7(1):376. doi: 10.1038/s41392-022-01191-9. Signal Transduct Target Ther. 2022. PMID: 36347846 Free PMC article. Review.
-
A novel NF2 splicing mutant causes neurofibromatosis type 2 via liquid-liquid phase separation with large tumor suppressor and Hippo pathway.iScience. 2022 Oct 4;25(11):105275. doi: 10.1016/j.isci.2022.105275. eCollection 2022 Nov 18. iScience. 2022. PMID: 36300003 Free PMC article.
-
Role of Merlin/NF2 inactivation in tumor biology.Oncogene. 2016 Feb 4;35(5):537-48. doi: 10.1038/onc.2015.125. Epub 2015 Apr 20. Oncogene. 2016. PMID: 25893302 Free PMC article. Review.
-
p120 RasGAP and ZO-2 are essential for Hippo signaling and tumor-suppressor function mediated by p190A RhoGAP.Cell Rep. 2023 Dec 26;42(12):113486. doi: 10.1016/j.celrep.2023.113486. Epub 2023 Nov 22. Cell Rep. 2023. PMID: 37995182 Free PMC article.
References
-
- Ahronowitz I, Xin W, Kiely R, Sims K, MacCollin M, Nunes FP. Mutational spectrum of the NF2 gene: a meta-analysis of 12 years of research and diagnostic laboratory findings. Hum Mutat. 2007;28:1–12. - PubMed
-
- Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol. 2002;3:586–599. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

