Calcium phosphate deposition rate, structure and osteoconductivity on electrospun poly(l-lactic acid) matrix using electrodeposition or simulated body fluid incubation
- PMID: 24012605
- PMCID: PMC3840094
- DOI: 10.1016/j.actbio.2013.08.041
Calcium phosphate deposition rate, structure and osteoconductivity on electrospun poly(l-lactic acid) matrix using electrodeposition or simulated body fluid incubation
Abstract
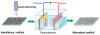
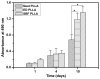
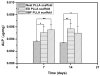
Mineralized nanofibrous scaffolds have been proposed as promising scaffolds for bone regeneration due to their ability to mimic both nanoscale architecture and chemical composition of natural bone extracellular matrix. In this study, a novel electrodeposition method was compared with an extensively explored simulated body fluid (SBF) incubation method in terms of the deposition rate, chemical composition and morphology of calcium phosphate formed on electrospun fibrous thin matrices with a fiber diameter in the range ~200-1400 nm prepared using 6, 8, 10 and 12 wt.% poly(l-lactic acid) (PLLA) solutions in a mixture of dichloromethane and acetone (2:1 in volume). The effects of the surface modification using the two mineralization techniques on osteoblastic cell (MC3T3-E1) proliferation and differentiation were also examined. It was found that electrodeposition was two to three orders of magnitude faster than the SBF method in mineralizing the fibrous matrices, reducing the mineralization time from ~2 weeks to 1h to achieve the same amounts of mineralization. The mineralization rate also varied with the fiber diameter but in opposite directions between the two mineralization methods. As a general trend, the increase of fiber diameter resulted in a faster mineralization rate for the electrodeposition method but a slower mineralization rate for the SBF incubation method. Using the electrodeposition method, one can control the chemical composition and morphology of the calcium phosphate by varying the electric deposition potential and electrolyte temperature to tune the mixture of dicalcium phosphate dihydrate and hydroxyapatite (HAp). Using the SBF method, one can only obtain a low crystallinity HAp. The mineralized electrospun PLLA fibrous matrices from either method similarly facilitate the proliferation and osteogenic differentiation of preosteoblastic MC3T3-E1 cells as compared to neat PLLA matrices. Therefore, the electrodeposition method can be utilized as a fast and versatile technique to fabricate mineralized nanofibrous scaffolds for bone tissue engineering.
Keywords: Bone tissue engineering; Calcium phosphate; Electrodeposition; Nanofiber; SBF incubation.
Copyright © 2013 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Figures








References
-
- Orban JM, Marra KG, Hollinger JO. Composition options for tissue-engineered bone. Tissue Eng. 2002;8(4):529–539. - PubMed
-
- Rezwan K, Chen QZ, Blaker JJ, Boccaccini AR. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials. 2006;27(18):3413–3431. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

