Young developmental age cardiac extracellular matrix promotes the expansion of neonatal cardiomyocytes in vitro
- PMID: 24012606
- PMCID: PMC3840040
- DOI: 10.1016/j.actbio.2013.08.037
Young developmental age cardiac extracellular matrix promotes the expansion of neonatal cardiomyocytes in vitro
Abstract
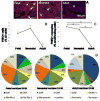
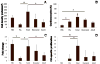
A major limitation to cardiac tissue engineering and regenerative medicine strategies is the lack of proliferation of postnatal cardiomyocytes. The extracellular matrix (ECM) is altered during heart development, and studies suggest that it plays an important role in regulating myocyte proliferation. Here, the effects of fetal, neonatal and adult cardiac ECM on the expansion of neonatal rat ventricular cells in vitro are studied. At 24h, overall cell attachment was lowest on fetal ECM; however, ~80% of the cells were cardiomyocytes, while many non-myocytes attached to older ECM and poly-l-lysine controls. After 5 days, the cardiomyocyte population remained highest on fetal ECM, with a 4-fold increase in number. Significantly more cardiomyocytes stained positively for the mitotic marker phospho-histone H3 on fetal ECM compared with other substrates at 5 days, suggesting that proliferation may be a major mechanism of cardiomyocyte expansion on young ECM. Further study of the beneficial properties of early developmental aged cardiac ECM could advance the design of novel biomaterials aimed at promoting cardiac regeneration.
Keywords: Cardiac tissue engineering; Cardiomyocyte; Extracellular matrix; Proliferation; Second Harmonic Generation imaging.
Copyright © 2013 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures





Similar articles
-
Partially Digested Adult Cardiac Extracellular Matrix Promotes Cardiomyocyte Proliferation In Vitro.Adv Healthc Mater. 2015 Jul 15;4(10):1545-54. doi: 10.1002/adhm.201500035. Epub 2015 May 18. Adv Healthc Mater. 2015. PMID: 25988681 Free PMC article.
-
Modulation of Mammalian Cardiomyocyte Cytokinesis by the Extracellular Matrix.Circ Res. 2020 Sep 11;127(7):896-907. doi: 10.1161/CIRCRESAHA.119.316303. Epub 2020 Jun 21. Circ Res. 2020. PMID: 32564729
-
Cardiomyocyte cell cycling, maturation, and growth by multinucleation in postnatal swine.J Mol Cell Cardiol. 2020 Sep;146:95-108. doi: 10.1016/j.yjmcc.2020.07.004. Epub 2020 Jul 22. J Mol Cell Cardiol. 2020. PMID: 32710980 Free PMC article.
-
Regeneration in heart disease-Is ECM the key?Life Sci. 2012 Oct 29;91(17-18):823-7. doi: 10.1016/j.lfs.2012.08.034. Epub 2012 Sep 12. Life Sci. 2012. PMID: 22982346 Free PMC article. Review.
-
The ECM as a driver of heart development and repair.Development. 2021 Mar 5;148(5):dev191320. doi: 10.1242/dev.191320. Development. 2021. PMID: 33674261 Review.
Cited by
-
Bioengineering approaches to mature induced pluripotent stem cell-derived atrial cardiomyocytes to model atrial fibrillation.Exp Biol Med (Maywood). 2021 Aug;246(16):1816-1828. doi: 10.1177/15353702211009146. Epub 2021 Apr 25. Exp Biol Med (Maywood). 2021. PMID: 33899540 Free PMC article. Review.
-
An Immunohistochemical Study of Proliferation of Human Fetal Heart Cardiomyocyte With Phospho-Histone H3 Antibody.Cureus. 2023 Jun 29;15(6):e41159. doi: 10.7759/cureus.41159. eCollection 2023 Jun. Cureus. 2023. PMID: 37525760 Free PMC article.
-
Extracellular Matrix Structure and Composition in the Early Four-Chambered Embryonic Heart.Cells. 2020 Jan 24;9(2):285. doi: 10.3390/cells9020285. Cells. 2020. PMID: 31991580 Free PMC article.
-
RNA sequencing indicates age-dependent shifts in the cardiac fibroblast transcriptome between fetal, neonatal, and adult developmental ages.Physiol Genomics. 2021 Oct 1;53(10):414-429. doi: 10.1152/physiolgenomics.00074.2021. Epub 2021 Jul 19. Physiol Genomics. 2021. PMID: 34281425 Free PMC article.
-
Opportunities for organoids as new models of aging.J Cell Biol. 2018 Jan 2;217(1):39-50. doi: 10.1083/jcb.201709054. Epub 2017 Dec 20. J Cell Biol. 2018. PMID: 29263081 Free PMC article. Review.
References
-
- Jenkins KJ, Correa A, Feinstein JA, Botto L, Britt AE, Daniels SR, et al. Noninherited risk factors and congenital cardiovascular defects: Current knowledge: A scientific statement from the American Heart Association council on cardiovascular disease in the young: Endorsed by the American Academy of Pediatrics. Circulation. 2007;115:2995–3014. - PubMed
-
- Stumper O. Hypoplastic left heart syndrome. Heart. 2010;96:231–6. - PubMed
-
- Fredenburg TB, Johnson TR, Cohen MD. The fontan procedure: anatomy, complications, and manifestations of failure. Radiographics. 2011;31:453–63. - PubMed
-
- Shin'oka T, Matsumara G, Hibino N, Naito Y, Watanabe M, Konuma T, et al. Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J Thorac Cardiovasc Surg. 2005;129:1330–2338. - PubMed
-
- Hibino N, McGillicuddy E, Matsumura G, Ichihara Y, Naito Y, Breuer C, et al. Late-term results of tissue-engineered vascular grafts in humans. J Thorac Cardiovasc Surg. 2010;139:431–6. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

