Activated alveolar epithelial cells initiate fibrosis through secretion of mesenchymal proteins
- PMID: 24012677
- PMCID: PMC3814683
- DOI: 10.1016/j.ajpath.2013.07.016
Activated alveolar epithelial cells initiate fibrosis through secretion of mesenchymal proteins
Abstract
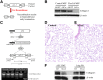
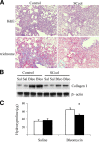
Fibrosis is characterized by accumulation of activated fibroblasts and pathological deposition of fibrillar collagens. Activated fibroblasts overexpress matrix proteins and release factors that promote further recruitment of activated fibroblasts, leading to progressive fibrosis. The contribution of epithelial cells to this process remains unknown. Epithelium-directed injury may lead to activation of epithelial cells with phenotypes and functions similar to activated fibroblasts. Prior reports that used a reporter gene fate-mapping strategy are limited in their ability to investigate the functional significance of epithelial cell-derived mesenchymal proteins during fibrogenesis. We found that lung epithelial cell-derived collagen I activates fibroblast collagen receptor discoidin domain receptor-2, contributes significantly to fibrogenesis, and promotes resolution of lung inflammation. Alveolar epithelial cells undergoing transforming growth factor-β-mediated mesenchymal transition express several other secreted profibrotic factors and are capable of activating lung fibroblasts. These studies provide direct evidence that activated epithelial cells produce mesenchymal proteins that initiate a cycle of fibrogenic effector cell activation, leading to progressive fibrosis. Therapy targeted at epithelial cell production of type I collagen offers a novel pathway for abrogating this progressive cycle and for limiting tissue fibrosis but may lead to sustained lung injury/inflammation.
Copyright © 2013 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Thannickal V.J., Toews G.B., White E.S., Lynch J.P., III, Martinez F.J. Mechanisms of pulmonary fibrosis. Annu Rev Med. 2004;55:395–417. - PubMed
-
- Selman M., King T.E., Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134:136–151. - PubMed
-
- American Thoracic Society Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS) Am J Respir Crit Care Med. 2000;161:646–664. - PubMed
-
- American Thoracic Society; European Respiratory Society American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165:277–304. - PubMed
-
- Scotton C.J., Chambers R.C. Molecular targets in pulmonary fibrosis: the myofibroblast in focus. Chest. 2007;132:1311–1321. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

