Endothelial Jagged-1 is necessary for homeostatic and regenerative hematopoiesis
- PMID: 24012753
- PMCID: PMC3805263
- DOI: 10.1016/j.celrep.2013.07.048
Endothelial Jagged-1 is necessary for homeostatic and regenerative hematopoiesis
Abstract
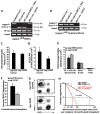
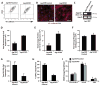
The bone marrow (BM) microenvironment is composed of multiple niche cells that, by producing paracrine factors, maintain and regenerate the hematopoietic stem cell (HSC) pool (Morrison and Spradling, 2008). We have previously demonstrated that endothelial cells support the proper regeneration of the hematopoietic system following myeloablation (Butler et al., 2010; Hooper et al., 2009; Kobayashi et al., 2010). Here, we demonstrate that expression of the angiocrine factor Jagged-1, supplied by the BM vascular niche, regulates homeostatic and regenerative hematopoiesis through a Notch-dependent mechanism. Conditional deletion of Jagged-1 in endothelial cells (Jag1((ECKO)) mice) results in a profound decrease in hematopoiesis and premature exhaustion of the adult HSC pool, whereas quantification and functional assays demonstrate that loss of Jagged-1 does not perturb vascular or mesenchymal compartments. Taken together, these data demonstrate that the instructive function of endothelial-specific Jagged-1 is required to support the self-renewal and regenerative capacity of HSCs in the adult BM vascular niche.
Copyright © 2013 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
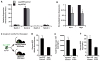
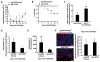

References
-
- Adams GB, Chabner KT, Alley IR, Olson DP, Szczepiorkowski ZM, Poznansky MC, Kos CH, Pollak MR, Brown EM, Scadden DT. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439:599–603. - PubMed
-
- Benedito R, Roca C, Sorensen I, Adams S, Gossler A, Fruttiger M, Adams RH. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137:1124–1135. - PubMed
-
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

