Single stimulus learning in zebrafish larvae
- PMID: 24012906
- PMCID: PMC3946257
- DOI: 10.1016/j.nlm.2013.08.016
Single stimulus learning in zebrafish larvae
Abstract
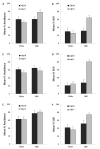
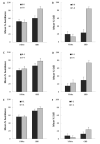
Learning about a moving visual stimulus was examined in zebrafish larvae using an automated imaging system and a t1-t2 design. In three experiments, zebrafish larvae were exposed to one of two inputs at t1 (either a gray bouncing disk or an identical but stationary disk) followed by a common test at t2 (the gray bouncing disk). Using 7days post-fertilization (dpf) larvae and 12 stimulus exposures, Experiment 1 established that these different treatments produced differential responding to the moving disk during testing. Larvae familiar with the moving test stimulus were significantly less likely to be still in its presence than larvae that had been exposed to the identical but stationary stimulus. Experiment 2 confirmed this result in 7dpf larvae and extended the finding to 5 and 6dpf larvae. Experiment 3 found differential responding to the moving test stimulus with 4 or 8 stimulus exposures but not with just one exposure in 7dpf larvae. These results provide evidence for learning in very young zebrafish larvae. The merits and challenges of the t1-t2 framework to study learning are discussed.
Keywords: Activity; Avoidance; Habituation; Learning; Thigmotaxis; Zebrafish larvae; t1–t2 Design.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures

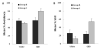
Similar articles
-
Effects of embryonic exposure to polychlorinated biphenyls (PCBs) on larval zebrafish behavior.Neurotoxicol Teratol. 2016 Jan-Feb;53:1-10. doi: 10.1016/j.ntt.2015.11.002. Epub 2015 Nov 10. Neurotoxicol Teratol. 2016. PMID: 26561944 Free PMC article.
-
Behavioral studies of stimulus learning in zebrafish larvae.Behav Processes. 2019 Jul;164:150-156. doi: 10.1016/j.beproc.2019.04.005. Epub 2019 May 2. Behav Processes. 2019. PMID: 31054948 Free PMC article. Review.
-
Comparability of behavioural assays using zebrafish larvae to assess neurotoxicity.Environ Sci Pollut Res Int. 2015 Nov;22(21):16277-89. doi: 10.1007/s11356-014-3805-8. Epub 2014 Nov 18. Environ Sci Pollut Res Int. 2015. PMID: 25399529 Review.
-
A novel high-throughput imaging system for automated analyses of avoidance behavior in zebrafish larvae.Behav Brain Res. 2011 Sep 30;223(1):135-44. doi: 10.1016/j.bbr.2011.04.033. Epub 2011 Apr 28. Behav Brain Res. 2011. PMID: 21549762 Free PMC article.
-
Environmental enrichment decreases anxiety-like behavior in zebrafish larvae.Dev Psychobiol. 2022 Mar;64(3):e22255. doi: 10.1002/dev.22255. Dev Psychobiol. 2022. PMID: 35312057 Free PMC article.
Cited by
-
Larval zebrafish display dynamic learning of aversive stimuli in a constant visual surrounding.Learn Mem. 2021 Jun 15;28(7):228-238. doi: 10.1101/lm.053425.121. Print 2021 Jul. Learn Mem. 2021. PMID: 34131054 Free PMC article.
-
Effects of embryonic exposure to polychlorinated biphenyls (PCBs) on larval zebrafish behavior.Neurotoxicol Teratol. 2016 Jan-Feb;53:1-10. doi: 10.1016/j.ntt.2015.11.002. Epub 2015 Nov 10. Neurotoxicol Teratol. 2016. PMID: 26561944 Free PMC article.
-
Behavioral studies of stimulus learning in zebrafish larvae.Behav Processes. 2019 Jul;164:150-156. doi: 10.1016/j.beproc.2019.04.005. Epub 2019 May 2. Behav Processes. 2019. PMID: 31054948 Free PMC article. Review.
-
Comparability of behavioural assays using zebrafish larvae to assess neurotoxicity.Environ Sci Pollut Res Int. 2015 Nov;22(21):16277-89. doi: 10.1007/s11356-014-3805-8. Epub 2014 Nov 18. Environ Sci Pollut Res Int. 2015. PMID: 25399529 Review.
-
Effects of embryonic exposure to polychlorinated biphenyls (PCBs) on anxiety-related behaviors in larval zebrafish.Neurotoxicology. 2016 Mar;53:93-101. doi: 10.1016/j.neuro.2015.12.018. Epub 2015 Dec 31. Neurotoxicology. 2016. PMID: 26748073 Free PMC article.
References
-
- Best JD, Berghmans S, Hunt JJ, Clarke SC, Fleming A, Goldsmith P, Roach AG. Non-associative learning in larval zebrafish. Neuropsychopharmacology. 2008;33(5):1206–1215. doi:10.1038/sj.npp.1301489. - PubMed
-
- Bilotta J. Effects of abnormal lighting on the development of zebrafish visual behavior. Behavioral Brain Research. 2000;116(1):81–87. doi: 10.1016/S0166-4328(00)00264-3. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

