High incidence of female reproductive tract cancers in FA-deficient HPV16-transgenic mice correlates with E7's induction of DNA damage response, an activity mediated by E7's inactivation of pocket proteins
- PMID: 24013229
- PMCID: PMC3999289
- DOI: 10.1038/onc.2013.327
High incidence of female reproductive tract cancers in FA-deficient HPV16-transgenic mice correlates with E7's induction of DNA damage response, an activity mediated by E7's inactivation of pocket proteins
Abstract
Fanconi anemia (FA) is a rare genetic disorder caused by defects in a DNA damage repair system, the FA pathway. FA patients frequently develop squamous cell carcinoma (SCC) at sites that are associated with human papillomavirus (HPV)-driven cancer including the female reproductive tract. To assess experimentally whether FA deficiency increases susceptibility to HPV-associated cervical/vaginal cancer, we monitored cancer incidence in the female lower reproductive tract of FA-deficient mice expressing HPV16 oncogenes, E6 and/or E7. FA deficiency specifically increased the incidence of cancers in mice expressing E7; but this effect was not observed in mice just expressing E6. We also observed that E7, but not E6, induced DNA damage as scored by induction of γ-H2AX and 53BP1 (p53 binding protein 1) nuclear foci, and this induction was heightened in FA-deficient tissue. Finally, we discovered that this induction of DNA damage responses was recapitulated in mice deficient in expression of 'pocket' proteins, pRb, p107 and p130, which are established targets of E7. Our findings support the hypothesis that E7 induces cancer by causing DNA damage at least in part through the inactivation of pocket proteins. This hypothesis explains why a deficiency in DNA damage repair would increase susceptibility to E7-driven cancer.
Figures
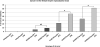





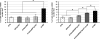
Similar articles
-
High incidence of HPV-associated head and neck cancers in FA deficient mice is associated with E7's induction of DNA damage through its inactivation of pocket proteins.PLoS One. 2013 Sep 23;8(9):e75056. doi: 10.1371/journal.pone.0075056. eCollection 2013. PLoS One. 2013. PMID: 24086435 Free PMC article.
-
Loss of Dependence on Continued Expression of the Human Papillomavirus 16 E7 Oncogene in Cervical Cancers and Precancerous Lesions Arising in Fanconi Anemia Pathway-Deficient Mice.mBio. 2016 May 17;7(3):e00628-16. doi: 10.1128/mBio.00628-16. mBio. 2016. PMID: 27190216 Free PMC article.
-
Repression of human papillomavirus oncogenes in HeLa cervical carcinoma cells causes the orderly reactivation of dormant tumor suppressor pathways.Proc Natl Acad Sci U S A. 2000 Nov 7;97(23):12513-8. doi: 10.1073/pnas.97.23.12513. Proc Natl Acad Sci U S A. 2000. PMID: 11070078 Free PMC article.
-
A novel MLL5 isoform that is essential to activate E6 and E7 transcription in HPV16/18-associated cervical cancers.Cancer Res. 2011 Nov 1;71(21):6696-707. doi: 10.1158/0008-5472.CAN-11-1271. Epub 2011 Sep 9. Cancer Res. 2011. PMID: 21908553
-
Warts, cancer and ubiquitylation: lessons from the papillomaviruses.Trans Am Clin Climatol Assoc. 2006;117:113-26; discussion 126-7. Trans Am Clin Climatol Assoc. 2006. PMID: 18528468 Free PMC article. Review.
Cited by
-
High-risk human papillomavirus oncogenes disrupt the Fanconi anemia DNA repair pathway by impairing localization and de-ubiquitination of FancD2.PLoS Pathog. 2019 Feb 28;15(2):e1007442. doi: 10.1371/journal.ppat.1007442. eCollection 2019 Feb. PLoS Pathog. 2019. PMID: 30818369 Free PMC article.
-
Cooperation of genes in HPV16 E6/E7-dependent cervicovaginal carcinogenesis trackable by endoscopy and independent of exogenous estrogens or carcinogens.Carcinogenesis. 2020 Nov 13;41(11):1605-1615. doi: 10.1093/carcin/bgaa027. Carcinogenesis. 2020. PMID: 32221533 Free PMC article.
-
Malignant Transformation of Fanconi Anemia Complementation Group D2-deficient (Fancd2 -/-) Hematopoietic Progenitor Cells by a Single HPV16 Oncogene.In Vivo. 2019 Mar-Apr;33(2):303-311. doi: 10.21873/invivo.11476. In Vivo. 2019. PMID: 30804107 Free PMC article.
-
FANCD2 limits acetaldehyde-induced genomic instability during DNA replication in esophageal keratinocytes.Mol Oncol. 2021 Nov;15(11):3109-3124. doi: 10.1002/1878-0261.13072. Epub 2021 Aug 8. Mol Oncol. 2021. PMID: 34328261 Free PMC article.
-
Head and Neck Cancer Susceptibility and Metabolism in Fanconi Anemia.Cancers (Basel). 2022 Apr 18;14(8):2040. doi: 10.3390/cancers14082040. Cancers (Basel). 2022. PMID: 35454946 Free PMC article. Review.
References
-
- Kutler DI, Singh B, Satagopan J, Batish SD, Berwick M, Giampietro PF, et al. A 20-year perspective on the International Fanconi Anemia Registry (IFAR). Blood. 2003 Feb 15;101(4):1249–56. - PubMed
-
- Joenje H, Patel KJ. The emerging genetic and molecular basis of Fanconi anaemia. Nat Rev Genet. 2001 Jun;2(6):446–57. - PubMed
-
- Crossan GP, Patel KJ. The Fanconi anaemia pathway orchestrates incisions at sites of crosslinked DNA. J Pathol. [Research Support, Non-U.S. Gov't Review] 2012 Jan;226(2):326–37. - PubMed
-
- Kennedy RD, D'Andrea AD. The Fanconi Anemia/BRCA pathway: new faces in the crowd. Genes Dev. 2005 Dec 15;19(24):2925–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

