D-alanine modification of a protease-susceptible outer membrane component by the Bordetella pertussis dra locus promotes resistance to antimicrobial peptides and polymorphonuclear leukocyte-mediated killing
- PMID: 24013634
- PMCID: PMC3811601
- DOI: 10.1128/JB.00510-13
D-alanine modification of a protease-susceptible outer membrane component by the Bordetella pertussis dra locus promotes resistance to antimicrobial peptides and polymorphonuclear leukocyte-mediated killing
Abstract
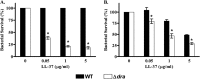
Bordetella pertussis is the causative agent of pertussis, a highly contagious disease of the human respiratory tract. Despite very high vaccine coverage, pertussis has reemerged as a serious threat in the United States and many developing countries. Thus, it is important to pursue research to discover unknown pathogenic mechanisms of B. pertussis. We have investigated a previously uncharacterized locus in B. pertussis, the dra locus, which is homologous to the dlt operons of Gram-positive bacteria. The absence of the dra locus resulted in increased sensitivity to the killing action of antimicrobial peptides (AMPs) and human phagocytes. Compared to the wild-type cells, the mutant cells bound higher levels of cationic proteins and peptides, suggesting that dra contributes to AMP resistance by decreasing the electronegativity of the cell surface. The presence of dra led to the incorporation of d-alanine into an outer membrane component that is susceptible to proteinase K cleavage. We conclude that dra encodes a virulence-associated determinant and contributes to the immune resistance of B. pertussis. With these findings, we have identified a new mechanism of surface modification in B. pertussis which may also be relevant in other Gram-negative pathogens.
Figures





Similar articles
-
Bordetella pertussis lipid A glucosamine modification confers resistance to cationic antimicrobial peptides and increases resistance to outer membrane perturbation.Antimicrob Agents Chemother. 2014 Aug;58(8):4931-4. doi: 10.1128/AAC.02590-14. Epub 2014 May 27. Antimicrob Agents Chemother. 2014. PMID: 24867963 Free PMC article.
-
Cloning and sequencing of a Bordetella pertussis serum resistance locus.Infect Immun. 1994 Nov;62(11):4727-38. doi: 10.1128/iai.62.11.4727-4738.1994. Infect Immun. 1994. PMID: 7927748 Free PMC article.
-
A recombinant iron transport protein from Bordetella pertussis confers protection against Bordetella parapertussis.Microbiol Immunol. 2017 Oct;61(10):407-415. doi: 10.1111/1348-0421.12532. Epub 2017 Sep 26. Microbiol Immunol. 2017. PMID: 28857261
-
Bordetella pertussis and pertactin-deficient clinical isolates: lessons for pertussis vaccines.Expert Rev Vaccines. 2014 Sep;13(9):1135-46. doi: 10.1586/14760584.2014.932254. Epub 2014 Jun 23. Expert Rev Vaccines. 2014. PMID: 24953157 Review.
-
Environmental sensing mechanisms in Bordetella.Adv Microb Physiol. 2001;44:141-81. doi: 10.1016/s0065-2911(01)44013-6. Adv Microb Physiol. 2001. PMID: 11407112 Review.
Cited by
-
Daptomycin Tolerance in the Staphylococcus aureus pitA6 Mutant Is Due to Upregulation of the dlt Operon.Antimicrob Agents Chemother. 2016 Apr 22;60(5):2684-91. doi: 10.1128/AAC.03022-15. Print 2016 May. Antimicrob Agents Chemother. 2016. PMID: 26883712 Free PMC article.
-
Bacterial strategies of resistance to antimicrobial peptides.Philos Trans R Soc Lond B Biol Sci. 2016 May 26;371(1695):20150292. doi: 10.1098/rstb.2015.0292. Philos Trans R Soc Lond B Biol Sci. 2016. PMID: 27160595 Free PMC article. Review.
-
Antimicrobial Peptide Resistance Genes in the Plant Pathogen Dickeya dadantii.Appl Environ Microbiol. 2016 Oct 14;82(21):6423-6430. doi: 10.1128/AEM.01757-16. Print 2016 Nov 1. Appl Environ Microbiol. 2016. PMID: 27565623 Free PMC article.
-
Genome Analysis of Enterobacter asburiae and Lelliottia spp. Proliferating in Oligotrophic Drinking Water Reservoirs and Lakes.Appl Environ Microbiol. 2022 Jul 26;88(14):e0047122. doi: 10.1128/aem.00471-22. Epub 2022 Jul 11. Appl Environ Microbiol. 2022. PMID: 35862664 Free PMC article.
-
Bordetella pertussis lipid A glucosamine modification confers resistance to cationic antimicrobial peptides and increases resistance to outer membrane perturbation.Antimicrob Agents Chemother. 2014 Aug;58(8):4931-4. doi: 10.1128/AAC.02590-14. Epub 2014 May 27. Antimicrob Agents Chemother. 2014. PMID: 24867963 Free PMC article.
References
-
- Centers for Disease Control and Prevention 2002. Pertussis—United States, 1997-2000. JAMA 287:977–979 - PubMed
-
- Centers for Disease Control and Prevention 2012. Pertussis epidemic—Washington, 2012. MMWR Morb. Mortal. Wkly. Rep. 61:517–522 - PubMed
-
- Mooi FR. 2010. Bordetella pertussis and vaccination: the persistence of a genetically monomorphic pathogen. Infect. Genet. Evol. 10:36–49 - PubMed
-
- McNabb SJ, Jajosky RA, Hall-Baker PA, Adams DA, Sharp P, Anderson WJ, Javier AJ, Jones GJ, Nitschke DA, Worshams CA, Richard RA., Jr 2007. Summary of notifiable diseases—United States, 2005. MMWR Morb. Mortal. Wkly. Rep. 54:1–92 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

