Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled Phase 3 study
- PMID: 24013647
- PMCID: PMC3888598
- DOI: 10.1136/annrheumdis-2013-204231
Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled Phase 3 study
Abstract
Objectives: To evaluate the efficacy and safety of certolizumab pegol (CZP) after 24 weeks in RAPID-axSpA (NCT01087762), an ongoing Phase 3 trial in patients with axial spondyloarthritis (axSpA), including patients with ankylosing spondylitis (AS) and non-radiographic axSpA (nr-axSpA).
Methods: Patients with active axSpA were randomised 1:1:1 to placebo, CZP 200 mg every 2 weeks (Q2W) or CZP 400 mg every 4 weeks (Q4W). In total 325 patients were randomised. Primary endpoint was ASAS20 (Assessment of SpondyloArthritis international Society 20) response at week 12. Secondary outcomes included change from baseline in Bath Ankylosing Spondylitis Functional Index (BASFI), Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), and Bath Ankylosing Spondylitis Metrology Index (BASMI) linear.
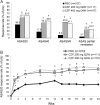
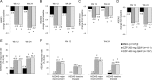
Results: Baseline disease activity was similar between AS and nr-axSpA. At week 12, ASAS20 response rates were significantly higher in CZP 200 mg Q2W and CZP 400 mg Q4W arms versus placebo (57.7 and 63.6 vs 38.3, p≤0.004). At week 24, combined CZP arms showed significant (p<0.001) differences in change from baseline versus placebo in BASFI (-2.28 vs -0.40), BASDAI (-3.05 vs -1.05), and BASMI (-0.52 vs -0.07). Improvements were observed as early as week 1. Similar improvements were reported with CZP versus placebo in both AS and nr-axSpA subpopulations. Adverse events were reported in 70.4% vs 62.6%, and serious adverse events in 4.7% vs 4.7% of All CZP versus placebo groups. No deaths or malignancies were reported.
Conclusions: CZP rapidly reduced the signs and symptoms of axSpA, with no new safety signals observed compared to the safety profile of CZP in RA. Similar improvements were observed across CZP dosing regimens, and in AS and nr-axSpA patients.
Figures


References
-
- Sieper J, Rudwaleit M, Baraliakos X, et al. The Assessment of SpondyloArthritis international Society (ASAS) handbook: a guide to assess spondyloarthritis. Ann Rheum Dis 2009;68(Suppl 2):ii1–44 - PubMed
-
- van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 1984;27:361–8 - PubMed
-
- Rudwaleit M, Landewé R, van der Heijde D, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part I): classification of paper patients by expert opinion including uncertainty appraisal. Ann Rheum Dis 2009;68:770–6 - PubMed
-
- Rudwaleit M, van der Heijde D, Landewé R, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009;68:777–83 - PubMed
-
- van den Berg R, de Hooge M, Rudwaleit M, et al. ASAS modification of the Berlin algorithm for diagnosing axial spondyloarthritis: results from the SPondyloArthritis Caught Early (SPACE)-cohort and from the Assessment of SpondyloArthritis international Society (ASAS)-cohort. Ann Rheum Dis 2013;72:1646–53 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

