A novel selective small-molecule PI3K inhibitor is effective against human multiple myeloma in vitro and in vivo
- PMID: 24013662
- PMCID: PMC3789203
- DOI: 10.1038/bcj.2013.37
A novel selective small-molecule PI3K inhibitor is effective against human multiple myeloma in vitro and in vivo
Abstract
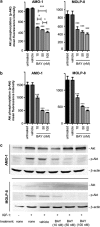
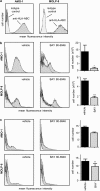
Developing effective therapies against multiple myeloma (MM) is an unresolved challenge. Phosphatidylinositol-3-kinase (PI3K) activation may be associated with tumor progression and drug resistance, and inhibiting PI3K can induce apoptosis in MM cells. Thus, targeting of PI3K is predicted to increase the susceptibility of MM to anticancer therapy. The lead compound of a novel class of PI3K inhibitors, BAY80-6946 (IC50=0.5 nM against PI3K-α), was highly efficacious in four different MM cell lines, where it induced significant antitumoral effects in a dose-dependent manner. The compound inhibited cell cycle progression and increased apoptosis (P<0.001 compared with controls). Moreover, it abrogated the stimulation conferred by insulin-like growth-factor-1, a mechanism relevant for MM progression. These cellular effects were paralleled by decreased Akt phosphorylation, the main downstream target of PI3K. Likewise, profound antitumoral activity was observed ex vivo, as BAY80-6946 significantly inhibited proliferation of freshly isolated myeloma cells from three patients (P<0.001 compared with vehicle). In addition, BAY80-6946 showed convincing in vivo activity against the human AMO-1 and MOLP-8 myeloma cell lines in a preclinical murine xenograft model, where treatment with 6 mg/kg every other day for 2 weeks reduced the cell numbers by 87.0% and 69.3%, respectively (P<0.001 compared with vehicle), without overt toxicity in treated animals.
Figures




References
-
- Raab MS, Podar K, Breitkreutz I, Richardson PG, Anderson KC. Multiple myeloma. Lancet. 2009;374:324–339. - PubMed
-
- Attal M, Harousseau J-L, Facon T, Guilhot F, Doyen C, Fuzibet JG, et al. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med. 2003;349:2495–2505. - PubMed
-
- Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004;351:1860–1873. - PubMed
-
- Lenhoff S, Hjorth M, Holmberg E, Turesson I, Westin J, Nielsen JL, et al. Impact on survival of high-dose therapy with autologous stem cell support in patients younger than 60 years with newly diagnosed multiple myeloma: a population-based study. Nordic Myeloma Study Group. Blood. 2000;95:7–11. - PubMed
-
- Richardson P, Hideshima T, Anderson KC. An update of novel therapeutic approaches for multiple myeloma. Curr Treat Options Oncol. 2004;5:227–238. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

