O-Linked β-N-acetylglucosamine (O-GlcNAc) regulates emerin binding to barrier to autointegration factor (BAF) in a chromatin- and lamin B-enriched "niche"
- PMID: 24014020
- PMCID: PMC3798487
- DOI: 10.1074/jbc.M113.503060
O-Linked β-N-acetylglucosamine (O-GlcNAc) regulates emerin binding to barrier to autointegration factor (BAF) in a chromatin- and lamin B-enriched "niche"
Abstract
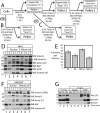
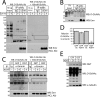
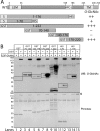
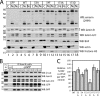
Emerin, a membrane component of nuclear "lamina" networks with lamins and barrier to autointegration factor (BAF), is highly O-GlcNAc-modified ("O-GlcNAcylated") in mammalian cells. Mass spectrometry analysis revealed eight sites of O-GlcNAcylation, including Ser-53, Ser-54, Ser-87, Ser-171, and Ser-173. Emerin O-GlcNAcylation was reduced ~50% by S53A or S54A mutation in vitro and in vivo. O-GlcNAcylation was reduced ~66% by the triple S52A/S53A/S54A mutant, and S173A reduced O-GlcNAcylation of the S52A/S53A/S54A mutant by ~30%, in vivo. We separated two populations of emerin, A-type lamins and BAF; one population solubilized easily, and the other required sonication and included histones and B-type lamins. Emerin and BAF associated only in histone- and lamin-B-containing fractions. The S173D mutation specifically and selectively reduced GFP-emerin association with BAF by 58% and also increased GFP-emerin hyper-phosphorylation. We conclude that β-N-acetylglucosaminyltransferase, an essential enzyme, controls two regions in emerin. The first region, defined by residues Ser-53 and Ser-54, flanks the LEM domain. O-GlcNAc modification at Ser-173, in the second region, is proposed to promote emerin association with BAF in the chromatin/lamin B "niche." These results reveal direct control of a conserved LEM domain nuclear lamina component by β-N-acetylglucosaminyltransferase, a nutrient sensor that regulates cell stress responses, mitosis, and epigenetics.
Keywords: Cardiomyopathy; Chromosomes/Non-histone Chromosomal Proteins; Emerin; Lamin; Laminopathy; Muscular Dystrophy; Nuclear Matrix; Nuclear Membrane; Nucleoskeleton; O-GlcNAc.
Figures


References
-
- Simon D. N., Wilson K. L. (2011) The nucleoskeleton as a genome-associated dynamic “network of networks”. Nat. Rev. Mol. Cell Biol. 12, 695–708 - PubMed
-
- Wagner N., Krohne G. (2007) LEM-domain proteins. New insights into lamin-interacting proteins. Int. Rev. Cytol. 261, 1–46 - PubMed
-
- Haraguchi T., Koujin T., Segura-Totten M., Lee K. K., Matsuoka Y., Yoneda Y., Wilson K. L., Hiraoka Y. (2001) BAF is required for emerin assembly into the reforming nuclear envelope. J. Cell Sci. 114, 4575–4585 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

