The nitric-oxide reductase from Paracoccus denitrificans uses a single specific proton pathway
- PMID: 24014024
- PMCID: PMC3798533
- DOI: 10.1074/jbc.M113.497347
The nitric-oxide reductase from Paracoccus denitrificans uses a single specific proton pathway
Abstract
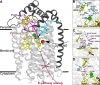
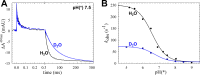
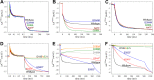
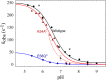
The NO reductase from Paracoccus denitrificans reduces NO to N2O (2NO + 2H(+) + 2e(-) → N2O + H2O) with electrons donated by periplasmic cytochrome c (cytochrome c-dependent NO reductase; cNOR). cNORs are members of the heme-copper oxidase superfamily of integral membrane proteins, comprising the O2-reducing, proton-pumping respiratory enzymes. In contrast, although NO reduction is as exergonic as O2 reduction, there are no protons pumped in cNOR, and in addition, protons needed for NO reduction are derived from the periplasmic solution (no contribution to the electrochemical gradient is made). cNOR thus only needs to transport protons from the periplasm into the active site without the requirement to control the timing of opening and closing (gating) of proton pathways as is needed in a proton pump. Based on the crystal structure of a closely related cNOR and molecular dynamics simulations, several proton transfer pathways were suggested, and in principle, these could all be functional. In this work, we show that residues in one of the suggested pathways (denoted pathway 1) are sensitive to site-directed mutation, whereas residues in the other proposed pathways (pathways 2 and 3) could be exchanged without severe effects on turnover activity with either NO or O2. We further show that electron transfer during single-turnover reduction of O2 is limited by proton transfer and can thus be used to study alterations in proton transfer rates. The exchange of residues along pathway 1 showed specific slowing of this proton-coupled electron transfer as well as changes in its pH dependence. Our results indicate that only pathway 1 is used to transfer protons in cNOR.
Keywords: Bioenergetics/Electron Transfer Complex; Electron Transfer; Enzyme Kinetics; Flow-Flash; Heme-Copper Oxidase; Kinetic Isotope Effect; Membrane Biophysics; Nitric Oxide; Proton Transport.
Figures
Similar articles
-
Investigating the Proton Donor in the NO Reductase from Paracoccus denitrificans.PLoS One. 2016 Mar 31;11(3):e0152745. doi: 10.1371/journal.pone.0152745. eCollection 2016. PLoS One. 2016. PMID: 27030968 Free PMC article.
-
The mechanism for oxygen reduction in cytochrome c dependent nitric oxide reductase (cNOR) as obtained from a combination of theoretical and experimental results.Biochim Biophys Acta Bioenerg. 2017 Nov;1858(11):884-894. doi: 10.1016/j.bbabio.2017.08.005. Epub 2017 Aug 8. Biochim Biophys Acta Bioenerg. 2017. PMID: 28801051
-
A pathway for protons in nitric oxide reductase from Paracoccus denitrificans.Biochim Biophys Acta. 2007 May;1767(5):362-73. doi: 10.1016/j.bbabio.2007.03.006. Epub 2007 Mar 16. Biochim Biophys Acta. 2007. PMID: 17466934
-
Proton transfer in bacterial nitric oxide reductase.Biochem Soc Trans. 2006 Feb;34(Pt 1):188-90. doi: 10.1042/BST0340188. Biochem Soc Trans. 2006. PMID: 16417518 Review.
-
The bacterial respiratory nitric oxide reductase.Biochem Soc Trans. 2009 Apr;37(Pt 2):392-9. doi: 10.1042/BST0370392. Biochem Soc Trans. 2009. PMID: 19290869 Review.
Cited by
-
Characterization of the quinol-dependent nitric oxide reductase from the pathogen Neisseria meningitidis, an electrogenic enzyme.Sci Rep. 2018 Feb 26;8(1):3637. doi: 10.1038/s41598-018-21804-0. Sci Rep. 2018. PMID: 29483528 Free PMC article.
-
Functional interactions between nitrite reductase and nitric oxide reductase from Paracoccus denitrificans.Sci Rep. 2019 Nov 21;9(1):17234. doi: 10.1038/s41598-019-53553-z. Sci Rep. 2019. PMID: 31754148 Free PMC article.
-
Mechanism of proton transfer through the KC proton pathway in the Vibrio cholerae cbb3 terminal oxidase.Biochim Biophys Acta Bioenerg. 2018 Nov;1859(11):1191-1198. doi: 10.1016/j.bbabio.2018.08.002. Epub 2018 Aug 22. Biochim Biophys Acta Bioenerg. 2018. PMID: 30251700 Free PMC article.
-
Investigating the Proton Donor in the NO Reductase from Paracoccus denitrificans.PLoS One. 2016 Mar 31;11(3):e0152745. doi: 10.1371/journal.pone.0152745. eCollection 2016. PLoS One. 2016. PMID: 27030968 Free PMC article.
-
Exploring long-range proton conduction, the conduction mechanism and inner hydration state of protein biopolymers.Chem Sci. 2020 Mar 11;11(13):3547-3556. doi: 10.1039/c9sc04392f. Chem Sci. 2020. PMID: 34109027 Free PMC article.
References
-
- Shiro Y. (2012) Structure and function of bacterial nitric oxide reductases. Nitric oxide reductase, anaerobic enzymes. Biochim. Biophys. Acta 1817, 1907–1913 - PubMed
-
- Lee H. J., Reimann J., Huang Y., Ädelroth P. (2012) Functional proton transfer pathways in the heme-copper oxidase superfamily. Biochim. Biophys. Acta 1817, 537–544 - PubMed
-
- Bell L. C., Richardson D. J., Ferguson S. J. (1992) Identification of nitric oxide reductase activity in Rhodobacter capsulatus. The electron transport pathway can either use or bypass both cytochrome c2 and the cytochrome bc1 complex. J. Gen. Microbiol. 138, 437–443 - PubMed
-
- Reimann J., Flock U., Lepp H., Honigmann A., Ädelroth P. (2007) A pathway for protons in nitric oxide reductase from Paracoccus denitrificans. Biochim. Biophys. Acta 1767, 362–373 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

