The thiamine biosynthetic enzyme ThiC catalyzes multiple turnovers and is inhibited by S-adenosylmethionine (AdoMet) metabolites
- PMID: 24014032
- PMCID: PMC3798539
- DOI: 10.1074/jbc.M113.500280
The thiamine biosynthetic enzyme ThiC catalyzes multiple turnovers and is inhibited by S-adenosylmethionine (AdoMet) metabolites
Abstract
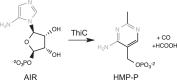
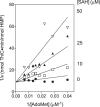
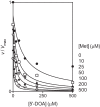
ThiC (4-amino-5-hydroxymethyl-2-methylpyrimidine phosphate synthase; EC 4.1.99.17) is a radical S-adenosylmethionine (AdoMet) enzyme that uses a [4Fe-4S](+) cluster to reductively cleave AdoMet to methionine and a 5'-deoxyadenosyl radical that initiates catalysis. In plants and bacteria, ThiC converts the purine intermediate 5-aminoimidazole ribotide to 4-amino-5-hydroxymethyl-2-methylpyrimidine phosphate, an intermediate of thiamine pyrophosphate (coenzyme B1) biosynthesis. In this study, assay conditions were implemented that consistently generated 5-fold molar excess of HMP, demonstrating that ThiC undergoes multiple turnovers. ThiC activity was improved by in situ removal of product 5'-deoxyadenosine. The activity was inhibited by AdoMet metabolites S-adenosylhomocysteine, adenosine, 5'-deoxyadenosine, S-methyl-5'-thioadenosine, methionine, and homocysteine. Neither adenosine nor S-methyl-5'-thioadenosine had been shown to inhibit radical AdoMet enzymes, suggesting that ThiC is distinct from other family members. The parameters for improved ThiC activity and turnover described here will facilitate kinetic and mechanistic analyses of ThiC.
Keywords: Enzyme Inhibitors; Iron-Sulfur Protein; Radicals; S-adenosylmethionine (AdoMet); Thiamine; Thiamine Biosynthesis.
Figures



Similar articles
-
ThiC is an [Fe-S] cluster protein that requires AdoMet to generate the 4-amino-5-hydroxymethyl-2-methylpyrimidine moiety in thiamin synthesis.Biochemistry. 2008 Sep 2;47(35):9054-6. doi: 10.1021/bi8010253. Epub 2008 Aug 8. Biochemistry. 2008. PMID: 18686975 Free PMC article.
-
Structural diversity in the AdoMet radical enzyme superfamily.Biochim Biophys Acta. 2012 Nov;1824(11):1178-95. doi: 10.1016/j.bbapap.2012.04.006. Epub 2012 Apr 28. Biochim Biophys Acta. 2012. PMID: 22579873 Free PMC article. Review.
-
Analysis of ThiC variants in the context of the metabolic network of Salmonella enterica.J Bacteriol. 2012 Nov;194(22):6088-95. doi: 10.1128/JB.01361-12. Epub 2012 Sep 7. J Bacteriol. 2012. PMID: 22961850 Free PMC article.
-
Reaction of AdoMet with ThiC generates a backbone free radical.Biochemistry. 2009 Jan 20;48(2):217-9. doi: 10.1021/bi802154j. Biochemistry. 2009. PMID: 19113839 Free PMC article.
-
Radical AdoMet enzymes in complex metal cluster biosynthesis.Biochim Biophys Acta. 2012 Nov;1824(11):1254-63. doi: 10.1016/j.bbapap.2012.01.002. Epub 2012 Jan 14. Biochim Biophys Acta. 2012. PMID: 22269887 Review.
Cited by
-
Radical Reaction Control in the AdoMet Radical Enzyme CDG Synthase (QueE): Consolidate, Destabilize, Accelerate.Chemistry. 2017 Jan 18;23(4):953-962. doi: 10.1002/chem.201604719. Epub 2016 Dec 13. Chemistry. 2017. PMID: 27859789 Free PMC article.
-
Why pyridoxal phosphate could be a functional predecessor of thiamine pyrophosphate and speculations on a primordial metabolism.RSC Chem Biol. 2024 Apr 18;5(6):508-517. doi: 10.1039/d4cb00016a. eCollection 2024 Jun 5. RSC Chem Biol. 2024. PMID: 38846080 Free PMC article. Review.
-
Investigation of ( S)-(-)-Acidomycin: A Selective Antimycobacterial Natural Product That Inhibits Biotin Synthase.ACS Infect Dis. 2019 Apr 12;5(4):598-617. doi: 10.1021/acsinfecdis.8b00345. Epub 2019 Feb 4. ACS Infect Dis. 2019. PMID: 30652474 Free PMC article.
-
A bioactive molecule made by unusual salvage of radical SAM enzyme byproduct 5-deoxyadenosine blurs the boundary of primary and secondary metabolism.J Biol Chem. 2021 Jan-Jun;296:100621. doi: 10.1016/j.jbc.2021.100621. Epub 2021 Mar 31. J Biol Chem. 2021. PMID: 33811856 Free PMC article.
-
OsTH1 is a key player in thiamin biosynthesis in rice.Sci Rep. 2024 Jun 12;14(1):13591. doi: 10.1038/s41598-024-62326-2. Sci Rep. 2024. PMID: 38866808 Free PMC article.
References
-
- Begley T. P., Downs D. M., Ealick S. E., McLafferty F. W., Van Loon A. P., Taylor S., Campobasso N., Chiu H. J., Kinsland C., Reddick J. J., Xi J. (1999) Thiamin biosynthesis in prokaryotes. Arch. Microbiol. 171, 293–300 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

