Mitochondrially mediated integrin αIIbβ3 protein inactivation limits thrombus growth
- PMID: 24014035
- PMCID: PMC3798537
- DOI: 10.1074/jbc.M113.472688
Mitochondrially mediated integrin αIIbβ3 protein inactivation limits thrombus growth
Abstract
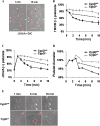
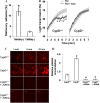
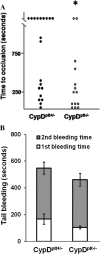
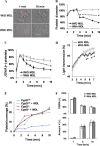
When platelets are strongly stimulated, a procoagulant platelet subpopulation is formed that is characterized by phosphatidylserine (PS) exposure and epitope modulation of integrin αIIbβ3 or a loss of binding of activation-dependent antibodies. Mitochondrial permeability transition pore (mPTP) formation, which is essential for the formation of procoagulant platelets, is impaired in the absence of cyclophilin D (CypD). Here we investigate the mechanisms responsible for these procoagulant platelet-associated changes in integrin αIIbβ3 and the physiologic role of procoagulant platelet formation in the regulation of platelet aggregation. Among strongly stimulated adherent platelets, integrin αIIbβ3 epitope changes, mPTP formation, PS exposure, and platelet rounding were closely associated. Furthermore, platelet mPTP formation resulted in a decreased ability to recruit additional platelets. In the absence of CypD, integrin αIIbβ3 function was accentuated in both static and flow conditions, and, in vivo, a prothrombotic phenotype occurred in mice with a platelet-specific deficiency of CypD. CypD-dependent proteolytic events, including cleavage of the integrin β3 cytoplasmic domain, coincided closely with integrin αIIbβ3 inactivation. Calpain inhibition blocked integrin β3 cleavage and inactivation but not mPTP formation or PS exposure, indicating that integrin inactivation and PS exposure are mediated by distinct pathways subsequent to mPTP formation. mPTP-dependent alkalinization occurred in procoagulant platelets, suggesting a possible alternative mechanism for enhancement of calpain activity in procoagulant platelets. Together, these results indicate that, in strongly stimulated platelets, mPTP formation initiates the calpain-dependent cleavage of integrin β3 and associated regulatory proteins, resulting in integrin αIIbβ3 inactivation, and demonstrate a novel CypD-dependent negative feedback mechanism that limits platelet aggregation and thrombotic occlusion.
Keywords: Calpain; Integrin; Mitochondria; Platelets; Thrombosis.
Figures


Similar articles
-
Dual mechanism of integrin αIIbβ3 closure in procoagulant platelets.J Biol Chem. 2013 May 10;288(19):13325-36. doi: 10.1074/jbc.M112.428359. Epub 2013 Mar 21. J Biol Chem. 2013. PMID: 23519467 Free PMC article.
-
Critical role for the mitochondrial permeability transition pore and cyclophilin D in platelet activation and thrombosis.Blood. 2008 Feb 1;111(3):1257-65. doi: 10.1182/blood-2007-05-092684. Epub 2007 Nov 7. Blood. 2008. PMID: 17989312 Free PMC article.
-
Small-Molecule Cyclophilin Inhibitors Potently Reduce Platelet Procoagulant Activity.Int J Mol Sci. 2023 Apr 12;24(8):7163. doi: 10.3390/ijms24087163. Int J Mol Sci. 2023. PMID: 37108326 Free PMC article.
-
Platelets and coagulation in thrombus formation: aberrations in the Scott syndrome.Thromb Res. 2016 May;141 Suppl 2:S12-6. doi: 10.1016/S0049-3848(16)30355-3. Thromb Res. 2016. PMID: 27207414 Review.
-
Procoagulant platelets and the pathways leading to cell death.Semin Thromb Hemost. 2015 Jun;41(4):405-12. doi: 10.1055/s-0034-1544002. Epub 2015 Apr 16. Semin Thromb Hemost. 2015. PMID: 26035696 Review.
Cited by
-
Circulating Platelets as Mediators of Immunity, Inflammation, and Thrombosis.Circ Res. 2018 Jan 19;122(2):337-351. doi: 10.1161/CIRCRESAHA.117.310795. Circ Res. 2018. PMID: 29348254 Free PMC article. Review.
-
Peripheral Blood Mononuclear Cells and Platelets Mitochondrial Dysfunction, Oxidative Stress, and Circulating mtDNA in Cardiovascular Diseases.J Clin Med. 2020 Jan 22;9(2):311. doi: 10.3390/jcm9020311. J Clin Med. 2020. PMID: 31979097 Free PMC article. Review.
-
Mechanisms Underlying Dichotomous Procoagulant COAT Platelet Generation-A Conceptual Review Summarizing Current Knowledge.Int J Mol Sci. 2022 Feb 25;23(5):2536. doi: 10.3390/ijms23052536. Int J Mol Sci. 2022. PMID: 35269679 Free PMC article. Review.
-
Inner Mitochondrial Membrane Disruption Links Apoptotic and Agonist-Initiated Phosphatidylserine Externalization in Platelets.Arterioscler Thromb Vasc Biol. 2017 Aug;37(8):1503-1512. doi: 10.1161/ATVBAHA.117.309473. Epub 2017 Jun 29. Arterioscler Thromb Vasc Biol. 2017. PMID: 28663253 Free PMC article.
-
IL-17 Induces MPTP opening through ERK2 and P53 signaling pathway in human platelets.J Huazhong Univ Sci Technolog Med Sci. 2015 Oct;35(5):679-683. doi: 10.1007/s11596-015-1489-z. Epub 2015 Oct 22. J Huazhong Univ Sci Technolog Med Sci. 2015. PMID: 26489621
References
-
- Dale G. L., Friese P., Batar P., Hamilton S. F., Reed G. L., Jackson K. W., Clemetson K. J., Alberio L. (2002) Stimulated platelets use serotonin to enhance their retention of procoagulant proteins on the cell surface. Nature 415, 175–179 - PubMed
-
- Munnix I. C., Cosemans J. M., Auger J. M., Heemskerk J. W. (2009) Platelet response heterogeneity in thrombus formation. Thromb. Haemost. 102, 1149–1156 - PubMed
-
- Alberio L., Safa O., Clemetson K. J., Esmon C. T., Dale G. L. (2000) Surface expression and functional characterization of α-granule factor V in human platelets. Effects of ionophore A23187, thrombin, collagen, and convulxin. Blood 95, 1694–1702 - PubMed
-
- Dale G. L. (2005) Coated-platelets. An emerging component of the procoagulant response. J. Thromb. Haemost 3, 2185–2192 - PubMed
-
- Heemskerk J. W., Vuist W. M., Feijge M. A., Reutelingsperger C. P., Lindhout T. (1997) Collagen but not fibrinogen surfaces induce bleb formation, exposure of phosphatidylserine, and procoagulant activity of adherent platelets. Evidence for regulation by protein tyrosine kinase-dependent Ca2+ responses. Blood 90, 2615–2625 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

