Longitudinal missing data strategies for substance use clinical trials using generalized estimating equations: an example with a buprenorphine trial
- PMID: 24014144
- PMCID: PMC3830126
- DOI: 10.1002/hup.2339
Longitudinal missing data strategies for substance use clinical trials using generalized estimating equations: an example with a buprenorphine trial
Abstract
Objective: A review of substance use clinical trials indicates that sub-optimal methods are the most commonly used procedures to deal with longitudinal missing information.
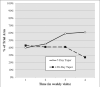
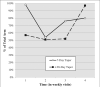
Methods: Listwise deletion (i.e., using complete cases only), positive urine analysis (UA) imputation, and multiple imputation (MI) were used to evaluate the effect of baseline substance use and buprenorphine/naloxone tapering schedule (7 or 28 days) on the probability of a positive UA (UA+) across the 4-week treatment period.
Results: The listwise deletion generalized estimating equations (GEE) model demonstrated that those in the 28-day taper group were less likely to submit a UA+ for opioids during the treatment period (odds ratios (OR) = 0.57, 95% confidence interval (CI): 0.39-0.83), as did the positive UA imputation model (OR = 0.43, CI: 0.34-0.55). The MI model also demonstrated a similar effect of taper group (OR = 0.57, CI: 0.42-0.77), but the effect size was more similar to that of the listwise deletion model.
Conclusions: Future researchers may find utilization of the MI procedure in conjunction with the common method of GEE analysis as a helpful analytic approach when the missing at random assumption is justifiable.
Keywords: generalized estimating equations; longitudinal missing data; multiple imputation; positive urine analysis imputation; psychopharmacology clinical trials; substance use disorder treatment.
Copyright © 2013 John Wiley & Sons, Ltd.
Figures
Similar articles
-
Missing data in substance abuse treatment research: current methods and modern approaches.Exp Clin Psychopharmacol. 2012 Jun;20(3):243-50. doi: 10.1037/a0027146. Epub 2012 Feb 13. Exp Clin Psychopharmacol. 2012. PMID: 22329556 Free PMC article.
-
A 'missing not at random' (MNAR) and 'missing at random' (MAR) growth model comparison with a buprenorphine/naloxone clinical trial.Addiction. 2015 Jan;110(1):51-8. doi: 10.1111/add.12714. Epub 2014 Oct 16. Addiction. 2015. PMID: 25170740 Free PMC article.
-
Imputation strategies for missing binary outcomes in cluster randomized trials.BMC Med Res Methodol. 2011 Feb 16;11:18. doi: 10.1186/1471-2288-11-18. BMC Med Res Methodol. 2011. PMID: 21324148 Free PMC article.
-
An overview of practical approaches for handling missing data in clinical trials.J Biopharm Stat. 2009 Nov;19(6):1055-73. doi: 10.1080/10543400903242795. J Biopharm Stat. 2009. PMID: 20183464 Review.
-
Analyzing longitudinal binary data in clinical studies.Contemp Clin Trials. 2022 Apr;115:106717. doi: 10.1016/j.cct.2022.106717. Epub 2022 Feb 28. Contemp Clin Trials. 2022. PMID: 35240309 Review.
Cited by
-
Predictors of cannabis and tobacco co-use in youth: exploring the mediating role of age at first use in the population assessment of tobacco health (PATH) study.J Cannabis Res. 2021 Jun 1;3(1):16. doi: 10.1186/s42238-021-00072-2. J Cannabis Res. 2021. PMID: 34074338 Free PMC article.
-
Predictors of tobacco and alcohol co-use from ages 15 to 32: The Amsterdam Growth and Health Longitudinal Study.Exp Clin Psychopharmacol. 2018 Dec;26(6):549-559. doi: 10.1037/pha0000203. Epub 2018 Aug 27. Exp Clin Psychopharmacol. 2018. PMID: 30148405 Free PMC article.
-
Empirically contrasting urine drug screening-based opioid use disorder treatment outcome definitions.Addiction. 2024 Jul;119(7):1289-1300. doi: 10.1111/add.16494. Epub 2024 Apr 14. Addiction. 2024. PMID: 38616571 Free PMC article.
-
Examining the factor structure of the Clinical Opiate Withdrawal Scale: A secondary data analysis from the National Drug Abuse Treatment Clinical Trials Network (CTN) 0003.Drug Alcohol Depend. 2015 Jul 1;152:218-23. doi: 10.1016/j.drugalcdep.2015.03.036. Epub 2015 Apr 11. Drug Alcohol Depend. 2015. PMID: 25908321 Free PMC article.
-
Parallel modeling of pain and depression in prediction of relapse during buprenorphine and naloxone treatment: A finite mixture model.Drug Alcohol Depend. 2020 Apr 1;209:107940. doi: 10.1016/j.drugalcdep.2020.107940. Epub 2020 Feb 26. Drug Alcohol Depend. 2020. PMID: 32135429 Free PMC article. Clinical Trial.
References
-
- Acock A. What to do about missing values. In: Cooper H, editor. APA Handbook of Research Methods in Psychology. American Psychological Association; Washington, DC: 2012.
-
- Allison PD. Missing Data. Sage; Newbury Park, CA: 2001.
-
- van Buuren S. Flexible Imputation of Missing Values. CRC Press; Boca Raton, FL: 2012.
-
- Collins LM, Schafer JL, Kam C- M. A comparison of inclusive and restrictive strategies in modern missing data procedures. Psychol Methods. 2001;6:330–351. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

