Modulation of Ca2+-currents by sequential and simultaneous activation of adenosine A1 and A 2A receptors in striatal projection neurons
- PMID: 24014158
- PMCID: PMC4040173
- DOI: 10.1007/s11302-013-9386-z
Modulation of Ca2+-currents by sequential and simultaneous activation of adenosine A1 and A 2A receptors in striatal projection neurons
Abstract
D(1)- and D(2)-types of dopamine receptors are located separately in direct and indirect pathway striatal projection neurons (dSPNs and iSPNs). In comparison, adenosine A(1)-type receptors are located in both neuron classes, and adenosine A(2A)-type receptors show a preferential expression in iSPNs. Due to their importance for neuronal excitability, Ca(2+)-currents have been used as final effectors to see the function of signaling cascades associated with different G protein-coupled receptors. For example, among many other actions, D(1)-type receptors increase, while D(2)-type receptors decrease neuronal excitability by either enhancing or reducing, respectively, CaV1 Ca(2+)-currents. These actions occur separately in dSPNs and iSPNs. In the case of purinergic signaling, the actions of A(1)- and A(2A)-receptors have not been compared observing their actions on Ca(2+)-channels of SPNs as final effectors. Our hypotheses are that modulation of Ca(2+)-currents by A(1)-receptors occurs in both dSPNs and iSPNs. In contrast, iSPNs would exhibit modulation by both A(1)- and A2A-receptors. We demonstrate that A(1)-type receptors reduced Ca(2+)-currents in all SPNs tested. However, A(2A)-type receptors enhanced Ca(2+)-currents only in half tested neurons. Intriguingly, to observe the actions of A(2A)-type receptors, occupation of A(1)-type receptors had to occur first. However, A(1)-receptors decreased Ca(V)2 Ca(2+)-currents, while A(2A)-type receptors enhanced current through Ca(V)1 channels. Because these channels have opposing actions on cell discharge, these differences explain in part why iSPNs may be more excitable than dSPNs. It is demonstrated that intrinsic voltage-gated currents expressed in SPNs are effectors of purinergic signaling that therefore play a role in excitability.
Figures

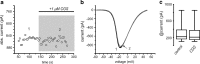

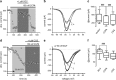

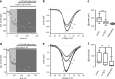


Similar articles
-
Modulation of direct pathway striatal projection neurons by muscarinic M₄-type receptors.Neuropharmacology. 2015 Feb;89:232-44. doi: 10.1016/j.neuropharm.2014.09.028. Epub 2014 Oct 5. Neuropharmacology. 2015. PMID: 25290553
-
Duration differences of corticostriatal responses in striatal projection neurons depend on calcium activated potassium currents.Front Syst Neurosci. 2013 Oct 4;7:63. doi: 10.3389/fnsys.2013.00063. eCollection 2013. Front Syst Neurosci. 2013. PMID: 24109439 Free PMC article.
-
The distinct role of medium spiny neurons and cholinergic interneurons in the D₂/A₂A receptor interaction in the striatum: implications for Parkinson's disease.J Neurosci. 2011 Feb 2;31(5):1850-62. doi: 10.1523/JNEUROSCI.4082-10.2011. J Neurosci. 2011. PMID: 21289195 Free PMC article.
-
Contribution of different classes of glutamate receptors in the corticostriatal polysynaptic responses from striatal direct and indirect projection neurons.BMC Neurosci. 2013 Jun 20;14:60. doi: 10.1186/1471-2202-14-60. BMC Neurosci. 2013. PMID: 23782743 Free PMC article.
-
Anatomy of adenosine A2A receptors in brain: morphological substrates for integration of striatal function.Neurology. 2003 Dec 9;61(11 Suppl 6):S12-8. doi: 10.1212/01.wnl.0000095205.33940.99. Neurology. 2003. PMID: 14663003 Review.
Cited by
-
Dopamine D2 and Adenosine A2A Receptors Interaction on Ca2+ Current Modulation in a Rodent Model of Parkinsonism.ASN Neuro. 2022 Jan-Dec;14:17590914221102075. doi: 10.1177/17590914221102075. ASN Neuro. 2022. PMID: 36050845 Free PMC article.
-
Calcium currents in striatal fast-spiking interneurons: dopaminergic modulation of CaV1 channels.BMC Neurosci. 2018 Jul 16;19(1):42. doi: 10.1186/s12868-018-0441-0. BMC Neurosci. 2018. PMID: 30012109 Free PMC article.
-
Adenosine A2A receptor blockade attenuates excitotoxicity in rat striatal medium spiny neurons during an ischemic-like insult.Neural Regen Res. 2024 Feb;19(2):255-257. doi: 10.4103/1673-5374.375309. Neural Regen Res. 2024. PMID: 37488874 Free PMC article. Review.
-
Cholinergic modulation of striatal microcircuits.Eur J Neurosci. 2019 Mar;49(5):604-622. doi: 10.1111/ejn.13949. Epub 2018 Nov 29. Eur J Neurosci. 2019. PMID: 29797362 Free PMC article. Review.
-
Histamine H3 receptor activation counteracts adenosine A2A receptor-mediated enhancement of depolarization-evoked [3H]-GABA release from rat globus pallidus synaptosomes.ACS Chem Neurosci. 2014 Aug 20;5(8):637-45. doi: 10.1021/cn500001m. Epub 2014 Jun 11. ACS Chem Neurosci. 2014. PMID: 24884070 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

