Inhibiting glutamine uptake represents an attractive new strategy for treating acute myeloid leukemia
- PMID: 24014241
- PMCID: PMC3829119
- DOI: 10.1182/blood-2013-03-493163
Inhibiting glutamine uptake represents an attractive new strategy for treating acute myeloid leukemia
Abstract
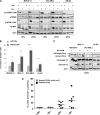
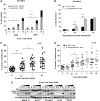
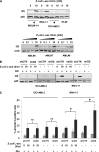
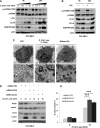
Cancer cells require nutrients and energy to adapt to increased biosynthetic activity, and protein synthesis inhibition downstream of mammalian target of rapamycin complex 1 (mTORC1) has shown promise as a possible therapy for acute myeloid leukemia (AML). Glutamine contributes to leucine import into cells, which controls the amino acid/Rag/mTORC1 signaling pathway. We show in our current study that glutamine removal inhibits mTORC1 and induces apoptosis in AML cells. The knockdown of the SLC1A5 high-affinity transporter for glutamine induces apoptosis and inhibits tumor formation in a mouse AML xenotransplantation model. l-asparaginase (l-ase) is an anticancer agent also harboring glutaminase activity. We show that l-ases from both Escherichia coli and Erwinia chrysanthemi profoundly inhibit mTORC1 and protein synthesis and that this inhibition correlates with their glutaminase activity levels and produces a strong apoptotic response in primary AML cells. We further show that l-ases upregulate glutamine synthase (GS) expression in leukemic cells and that a GS knockdown enhances l-ase-induced apoptosis in some AML cells. Finally, we observe a strong autophagic process upon l-ase treatment. These results suggest that l-ase anticancer activity and glutamine uptake inhibition are promising new therapeutic strategies for AML.
Figures


Comment in
-
Asparaginase unveils glutamine-addicted AML.Blood. 2013 Nov 14;122(20):3398-400. doi: 10.1182/blood-2013-09-526392. Blood. 2013. PMID: 24235130
References
-
- Tamburini J, Chapuis N, Bardet V, et al. Mammalian target of rapamycin (mTOR) inhibition activates phosphatidylinositol 3-kinase/Akt by up-regulating insulin-like growth factor-1 receptor signaling in acute myeloid leukemia: rationale for therapeutic inhibition of both pathways. Blood. 2008;111(1):379–382. - PubMed
-
- Dos Santos C, Demur C, Bardet V, Prade-Houdellier N, Payrastre B, Récher C. A critical role for Lyn in acute myeloid leukemia. Blood. 2008;111(4):2269–2279. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

