Neocortical neurogenesis and neuronal migration
- PMID: 24014417
- PMCID: PMC3767922
- DOI: 10.1002/wdev.88
Neocortical neurogenesis and neuronal migration
Abstract
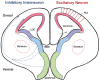
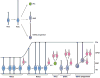
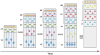
The neocortex, the evolutionarily newest part of the cerebral cortex, controls nearly all aspects of behavior, including perception, language, and decision making. It contains an immense number of neurons that can be broadly divided into two groups, excitatory neurons and inhibitory interneurons. These neurons are predominantly produced through extensive progenitor cell divisions during the embryonic stages. Moreover, they are not randomly dispersed, but spatially organized into horizontal layers that are essential for neocortex function. The formation of this laminar structure requires exquisite control of neuronal migration from their birthplace to their final destination. Extensive research over the past decade has greatly advanced our understanding of the production and migration of both excitatory neurons and inhibitory interneurons in the developing neocortex. In this review, we aim to give an overview on the molecular and cellular processes of neocortical neurogenesis and neuronal migration.
Copyright © 2012 Wiley Periodicals, Inc.
Figures


Similar articles
-
Clonal production and organization of inhibitory interneurons in the neocortex.Science. 2011 Oct 28;334(6055):480-6. doi: 10.1126/science.1208884. Science. 2011. PMID: 22034427 Free PMC article.
-
Involvement of SF-1 in neurogenesis and neuronal migration in the developing neocortex.Neurosci Lett. 2015 Jul 23;600:85-90. doi: 10.1016/j.neulet.2015.06.005. Epub 2015 Jun 9. Neurosci Lett. 2015. PMID: 26067405
-
Tangential migration and proliferation of intermediate progenitors of GABAergic neurons in the mouse telencephalon.Development. 2011 Jun;138(12):2499-509. doi: 10.1242/dev.063032. Epub 2011 May 11. Development. 2011. PMID: 21561989
-
Transcriptional co-regulation of neuronal migration and laminar identity in the neocortex.Development. 2012 May;139(9):1535-46. doi: 10.1242/dev.069963. Development. 2012. PMID: 22492350 Free PMC article. Review.
-
Lineage origins of GABAergic versus glutamatergic neurons in the neocortex.Curr Opin Neurobiol. 2014 Jun;26:132-41. doi: 10.1016/j.conb.2014.01.015. Epub 2014 Feb 16. Curr Opin Neurobiol. 2014. PMID: 24549207 Free PMC article. Review.
Cited by
-
N-acetylcysteine attenuates lipopolysaccharide-induced impairment in lamination of Ctip2-and Tbr1- expressing cortical neurons in the developing rat fetal brain.Sci Rep. 2016 Aug 31;6:32373. doi: 10.1038/srep32373. Sci Rep. 2016. PMID: 27577752 Free PMC article.
-
Advances in Understanding the Molecular Mechanisms of Neuronal Polarity.Mol Neurobiol. 2023 May;60(5):2851-2870. doi: 10.1007/s12035-023-03242-w. Epub 2023 Feb 4. Mol Neurobiol. 2023. PMID: 36738353 Review.
-
LIS1 and DCX: Implications for Brain Development and Human Disease in Relation to Microtubules.Scientifica (Cairo). 2013;2013:393975. doi: 10.1155/2013/393975. Epub 2013 Mar 17. Scientifica (Cairo). 2013. PMID: 24278775 Free PMC article. Review.
-
Cerebral organoids as tools to identify the developmental roots of autism.Mol Autism. 2020 Jul 13;11(1):58. doi: 10.1186/s13229-020-00360-3. Mol Autism. 2020. PMID: 32660622 Free PMC article. Review.
-
Calcium and Neural Stem Cell Proliferation.Int J Mol Sci. 2024 Apr 6;25(7):4073. doi: 10.3390/ijms25074073. Int J Mol Sci. 2024. PMID: 38612887 Free PMC article. Review.
References
-
- Gotz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005;6:777–788. - PubMed
-
- Williams BP, Price J. Evidence for multiple precursor cell types in the embryonic rat cerebral cortex. Neuron. 1995;14:1181–1188. - PubMed
-
- Hartfuss E, Galli R, Heins N, Gotz M. Characterization of CNS precursor subtypes and radial glia. Dev Biol. 2001;229:15–30. - PubMed
-
- Malatesta P, Hack MA, Hartfuss E, Kettenmann H, Klinkert W, Kirchhoff F, Gotz M. Neuronal or glial progeny: regional differences in radial glia fate. Neuron. 2003;37:751–764. - PubMed
-
- Aaku-Saraste E, Hellwig A, Huttner WB. Loss of occludin and functional tight junctions, but not ZO-1, during neural tube closure--remodeling of the neuroepithelium prior to neurogenesis. Dev Biol. 1996;180:664–679. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

