Genetic causes of microcephaly and lessons for neuronal development
- PMID: 24014418
- PMCID: PMC3767923
- DOI: 10.1002/wdev.89
Genetic causes of microcephaly and lessons for neuronal development
Abstract
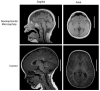
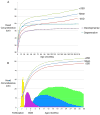
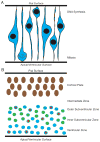
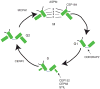
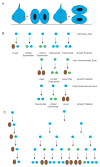
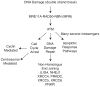
The study of human developmental microcephaly is providing important insights into brain development. It has become clear that developmental microcephalies are associated with abnormalities in cellular production, and that the pathophysiology of microcephaly provides remarkable insights into how the brain generates the proper number of neurons that determine brain size. Most of the genetic causes of 'primary' developmental microcephaly (i.e., not associated with other syndromic features) are associated with centrosomal abnormalities. In addition to other functions, centrosomal proteins control the mitotic spindle, which is essential for normal cell proliferation during mitosis. However, the brain is often uniquely affected when microcephaly genes are mutated implying special centrosomal-related functions in neuronal production. Although models explaining how this could occur have some compelling data, they are not without controversy. Interestingly, some of the microcephaly genes show evidence that they were targets of evolutionary selection in primates and human ancestors, suggesting potential evolutionary roles in controlling neuronal number and brain volume across species. Mutations in DNA repair pathway genes also lead to microcephaly. Double-stranded DNA breaks appear to be a prominent type of damage that needs to be repaired during brain development, yet why defects in DNA repair affect the brain preferentially and if DNA repair relates to centrosome function, are not clearly understood.
Copyright © 2012 Wiley Periodicals, Inc.
Figures
References
-
- Dolk H. The predictive value of microcephaly during the first year of life for mental retardation at seven years. Dev Med Child Neurol. 1991;33:974–983. - PubMed
-
- Yu TW, Mochida GH, Tischfield DJ, Sgaier SK, Flores-Sarnat L, Sergi CM, Topcu M, McDonald MT, Barry BJ, Felie JM, et al. Mutations in WDR62, encoding a centrosome-associated protein, cause microcephaly with simplified gyri and abnormal cortical architecture. Nat Genet. 2010;42:1015–1020. - PMC - PubMed
-
- Spalding KL, Bhardwaj RD, Buchholz BA, Druid H, Frisen J. Retrospective birth dating of cells in humans. Cell. 2005;122:133–143. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

