Cardiac outflow tract anomalies
- PMID: 24014420
- PMCID: PMC4021394
- DOI: 10.1002/wdev.98
Cardiac outflow tract anomalies
Abstract
The mature outflow tract (OFT) is, in basic terms, a short conduit. It is a simple, although vital, connection situated between contracting muscular heart chambers and a vast embryonic vascular network. Unfortunately, it is also a focal point underlying many multifactorial congenital heart defects (CHDs). Through the use of various animal models combined with human genetic investigations, we are beginning to comprehend the molecular and cellular framework that controls OFT morphogenesis. Clear roles of neural crest cells (NCC) and second heart field (SHF) derivatives have been established during OFT formation and remodeling. The challenge now is to determine how the SHF and cardiac NCC interact, the complex reciprocal signaling that appears to be occurring at various stages of OFT morphogenesis, and finally how endocardial progenitors and primary heart field (PHF) communicate with both these colonizing extra-cardiac lineages. Although we are beginning to understand that this dance of progenitor populations is wonderfully intricate, the underlying pathogenesis and the spatiotemporal cell lineage interactions remain to be fully elucidated. What is now clear is that OFT alignment and septation are independent processes, invested via separate SHF and cardiac neural crest (CNC) lineages. This review will focus on our current understanding of the respective contributions of the SHF and CNC lineage during OFT development and pathogenesis.
Copyright © 2013 Wiley Periodicals, Inc.
Figures

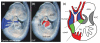
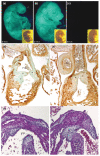

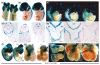

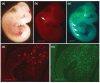
Similar articles
-
HAND transcription factors cooperatively specify the aorta and pulmonary trunk.Dev Biol. 2021 Aug;476:1-10. doi: 10.1016/j.ydbio.2021.03.011. Epub 2021 Mar 20. Dev Biol. 2021. PMID: 33757801 Free PMC article.
-
Rac1 Signaling Is Required for Anterior Second Heart Field Cellular Organization and Cardiac Outflow Tract Development.J Am Heart Assoc. 2015 Dec 31;5(1):e002508. doi: 10.1161/JAHA.115.002508. J Am Heart Assoc. 2015. PMID: 26722124 Free PMC article.
-
Cardiac Neural Crest.Cold Spring Harb Perspect Biol. 2021 Jan 4;13(1):a036715. doi: 10.1101/cshperspect.a036715. Cold Spring Harb Perspect Biol. 2021. PMID: 32071091 Free PMC article. Review.
-
LRP2 controls sonic hedgehog-dependent differentiation of cardiac progenitor cells during outflow tract formation.Hum Mol Genet. 2020 Nov 25;29(19):3183-3196. doi: 10.1093/hmg/ddaa200. Hum Mol Genet. 2020. PMID: 32901292 Free PMC article.
-
Molecular Pathways and Animal Models of Tetralogy of Fallot and Double Outlet Right Ventricle.Adv Exp Med Biol. 2024;1441:645-659. doi: 10.1007/978-3-031-44087-8_37. Adv Exp Med Biol. 2024. PMID: 38884739 Review.
Cited by
-
Hippo Signaling Mediates TGFβ-Dependent Transcriptional Inputs in Cardiac Cushion Mesenchymal Cells to Regulate Extracellular Matrix Remodeling.J Cardiovasc Dev Dis. 2023 Dec 4;10(12):483. doi: 10.3390/jcdd10120483. J Cardiovasc Dev Dis. 2023. PMID: 38132651 Free PMC article.
-
Drosophila as a Model to Understand Second Heart Field Development.J Cardiovasc Dev Dis. 2023 Dec 12;10(12):494. doi: 10.3390/jcdd10120494. J Cardiovasc Dev Dis. 2023. PMID: 38132661 Free PMC article. Review.
-
Targeted laser ablation of the zebrafish larval heart induces models of heart block, valvular regurgitation, and outflow tract obstruction.Zebrafish. 2014 Dec;11(6):536-41. doi: 10.1089/zeb.2014.1027. Zebrafish. 2014. PMID: 25272304 Free PMC article.
-
The role of glucose in physiological and pathological heart formation.Dev Biol. 2021 Jul;475:222-233. doi: 10.1016/j.ydbio.2021.01.020. Epub 2021 Feb 10. Dev Biol. 2021. PMID: 33577830 Free PMC article. Review.
-
The Role of Sorting Nexin 17 in Cardiac Development.Front Cardiovasc Med. 2021 Dec 20;8:748891. doi: 10.3389/fcvm.2021.748891. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34988124 Free PMC article.
References
-
- Mitchell SC, Korones SB, Berendes HW. Congenital heart disease in 56,109 births. Incidence and natural history. Circulation. 1971;43:323–332. - PubMed
-
- Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. - PubMed
-
- van der Linde D, Konings EE, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJ, Roos-Hesselink JW. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58:2241–2247. - PubMed
-
- Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, Zheng ZJ, Flegal K, O'Donnell C, Kittner S, et al. Heart disease and stroke statistics–2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–e151. - PubMed
-
- Conway SJ, Kruzynska-Frejtag A, Kneer PL, Machnicki M, Koushik SV. What cardiovascular defect does my prenatal mouse mutant have, and why? Genesis. 2003;35:1–21. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

