Unraveling the Leloir pathway of Bifidobacterium bifidum: significance of the uridylyltransferases
- PMID: 24014529
- PMCID: PMC3811521
- DOI: 10.1128/AEM.02460-13
Unraveling the Leloir pathway of Bifidobacterium bifidum: significance of the uridylyltransferases
Abstract
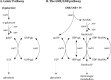
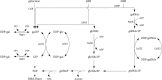
The GNB/LNB (galacto-N-biose/lacto-N-biose) pathway plays a crucial role in bifidobacteria during growth on human milk or mucin from epithelial cells. It is thought to be the major route for galactose utilization in Bifidobacterium longum as it is an energy-saving variant of the Leloir pathway. Both pathways are present in B. bifidum, and galactose 1-phosphate (gal1P) is considered to play a key role. Due to its toxic nature, gal1P is further converted into its activated UDP-sugar through the action of poorly characterized uridylyltransferases. In this study, three uridylyltransferases (galT1, galT2, and ugpA) from Bifidobacterium bifidum were cloned in an Escherichia coli mutant and screened for activity on the key intermediate gal1P. GalT1 and GalT2 showed UDP-glucose-hexose-1-phosphate uridylyltransferase activity (EC 2.7.7.12), whereas UgpA showed promiscuous UTP-hexose-1-phosphate uridylyltransferase activity (EC 2.7.7.10). The activity of UgpA toward glucose 1-phosphate was about 33-fold higher than that toward gal1P. GalT1, as part of the bifidobacterial Leloir pathway, was about 357-fold more active than GalT2, the functional analog in the GNB/LNB pathway. These results suggest that GalT1 plays a more significant role than previously thought and predominates when B. bifidum grows on lactose and human milk oligosaccharides. GalT2 activity is required only during growth on substrates with a GNB core such as mucin glycans.
Figures


Similar articles
-
Structure and evolution of the bifidobacterial carbohydrate metabolism proteins and enzymes.Biochem Soc Trans. 2021 Apr 30;49(2):563-578. doi: 10.1042/BST20200163. Biochem Soc Trans. 2021. PMID: 33666221 Free PMC article. Review.
-
Bifidobacterial enzymes involved in the metabolism of human milk oligosaccharides.Adv Nutr. 2012 May 1;3(3):422S-9S. doi: 10.3945/an.111.001420. Adv Nutr. 2012. PMID: 22585921 Free PMC article. Review.
-
Identification of N-acetylhexosamine 1-kinase in the complete lacto-N-biose I/galacto-N-biose metabolic pathway in Bifidobacterium longum.Appl Environ Microbiol. 2007 Oct;73(20):6444-9. doi: 10.1128/AEM.01425-07. Epub 2007 Aug 24. Appl Environ Microbiol. 2007. PMID: 17720833 Free PMC article.
-
Large scale production of lacto-N-biose I, a building block of type I human milk oligosaccharides, using sugar phosphorylases.Biosci Biotechnol Biochem. 2020 Jan;84(1):17-24. doi: 10.1080/09168451.2019.1670047. Epub 2019 Sep 28. Biosci Biotechnol Biochem. 2020. PMID: 31566084
-
Distribution of in vitro fermentation ability of lacto-N-biose I, a major building block of human milk oligosaccharides, in bifidobacterial strains.Appl Environ Microbiol. 2010 Jan;76(1):54-9. doi: 10.1128/AEM.01683-09. Epub 2009 Oct 23. Appl Environ Microbiol. 2010. PMID: 19854932 Free PMC article.
Cited by
-
Structure and evolution of the bifidobacterial carbohydrate metabolism proteins and enzymes.Biochem Soc Trans. 2021 Apr 30;49(2):563-578. doi: 10.1042/BST20200163. Biochem Soc Trans. 2021. PMID: 33666221 Free PMC article. Review.
-
A novel gene cluster allows preferential utilization of fucosylated milk oligosaccharides in Bifidobacterium longum subsp. longum SC596.Sci Rep. 2016 Oct 19;6:35045. doi: 10.1038/srep35045. Sci Rep. 2016. PMID: 27756904 Free PMC article.
-
Structure of co-expression networks of Bifidobacterium species in response to human milk oligosaccharides.Front Mol Biosci. 2023 Jan 26;10:1040721. doi: 10.3389/fmolb.2023.1040721. eCollection 2023. Front Mol Biosci. 2023. PMID: 36776740 Free PMC article.
-
Transcriptional Regulation of Carbohydrate Utilization Pathways in the Bifidobacterium Genus.Front Microbiol. 2016 Feb 9;7:120. doi: 10.3389/fmicb.2016.00120. eCollection 2016. Front Microbiol. 2016. PMID: 26903998 Free PMC article.
-
Milk glycan metabolism by intestinal bifidobacteria: insights from comparative genomics.Crit Rev Biochem Mol Biol. 2022 Oct-Dec;57(5-6):562-584. doi: 10.1080/10409238.2023.2182272. Epub 2023 Mar 3. Crit Rev Biochem Mol Biol. 2022. PMID: 36866565 Free PMC article. Review.
References
-
- Tissier H. 1900. Recherches sur la flore intestinale des nourrissons (état normal et pathologique). Ph.D. thesis University of Paris, Paris, France
-
- Guarner F, Malagelada JR. 2003. Gut flora in health and disease. Lancet 361:512–519 - PubMed
-
- Fushinobu S. 2010. Unique sugar metabolic pathways of bifidobacteria. Biosci. Biotechnol. Biochem. 74:2374–2384 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

