COX-1-derived PGE2 and PGE2 type 1 receptors are vital for angiotensin II-induced formation of reactive oxygen species and Ca(2+) influx in the subfornical organ
- PMID: 24014678
- PMCID: PMC3840259
- DOI: 10.1152/ajpheart.00238.2013
COX-1-derived PGE2 and PGE2 type 1 receptors are vital for angiotensin II-induced formation of reactive oxygen species and Ca(2+) influx in the subfornical organ
Abstract
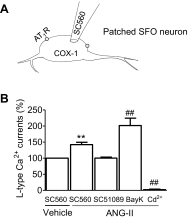
Regulation of blood pressure by angiotensin II (ANG II) is a process that involves the reactive oxygen species (ROS) and calcium. We have shown that ANG-II type 1 receptor (AT1R) and prostaglandin E2 (PGE2) type 1 receptors (EP1R) are required in the subfornical organ (SFO) for ROS-mediated hypertension induced by slow-pressor ANG-II infusion. However, the signaling pathway associated with this process remains unclear. We sought to determine mechanisms underlying the ANG II-induced ROS and calcium influx in mouse SFO cells. Ultrastructural studies showed that cyclooxygenase 1 (COX-1) codistributes with AT1R in the SFO, indicating spatial proximity. Functional studies using SFO cells revealed that ANG II potentiated PGE2 release, an effect dependent on AT1R, phospholipase A2 (PLA2) and COX-1. Furthermore, both ANG II and PGE2 increased ROS formation. While the increase in ROS initiated by ANG II, but not PGE2, required the activation of the AT1R/PLA2/COX-1 pathway, both ANG II and PGE2 were dependent on EP1R and Nox2 as downstream effectors. Finally, ANG II potentiated voltage-gated L-type Ca(2+) currents in SFO neurons via the same signaling pathway required for PGE2 production. Blockade of EP1R and Nox2-derived ROS inhibited ANG II and PGE2-mediated Ca(2+) currents. We propose a mechanism whereby ANG II increases COX-1-derived PGE2 through the AT1R/PLA2 pathway, which promotes ROS production by EP1R/Nox2 signaling in the SFO. ANG II-induced ROS are coupled with Ca(2+) influx in SFO neurons, which may influence SFO-mediated sympathoexcitation. Our findings provide the first evidence of a spatial and functional framework that underlies ANG-II signaling in the SFO and reveal novel targets for antihypertensive therapies.
Keywords: angiotensin II; calcium channel; hypertension; prostaglandins; reactive oxygen species; slow-pressor; subfornical organ.
Figures








References
-
- Anderson JW, Smith PM, Ferguson AV. Subfornical organ neurons projecting to paraventricular nucleus: whole-cell properties. Brain Res 921: 78–85, 2001 - PubMed
-
- Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature 391: 281–285, 1998 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

