Mutations on the N-terminal edge of the DELSEED loop in either the α or β subunit of the mitochondrial F1-ATPase enhance ATP hydrolysis in the absence of the central γ rotor
- PMID: 24014764
- PMCID: PMC3837939
- DOI: 10.1128/EC.00177-13
Mutations on the N-terminal edge of the DELSEED loop in either the α or β subunit of the mitochondrial F1-ATPase enhance ATP hydrolysis in the absence of the central γ rotor
Abstract
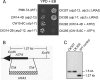
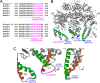
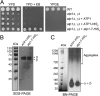
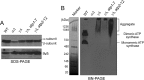
F(1)-ATPase is a rotary molecular machine with a subunit stoichiometry of α(3)β(3)γ(1)δ(1)ε(1). It has a robust ATP-hydrolyzing activity due to effective cooperativity between the three catalytic sites. It is believed that the central γ rotor dictates the sequential conformational changes to the catalytic sites in the α(3)β(3) core to achieve cooperativity. However, recent studies of the thermophilic Bacillus PS3 F(1)-ATPase have suggested that the α(3)β(3) core can intrinsically undergo unidirectional cooperative catalysis (T. Uchihashi et al., Science 333:755-758, 2011). The mechanism of this γ-independent ATP-hydrolyzing mode is unclear. Here, a unique genetic screen allowed us to identify specific mutations in the α and β subunits that stimulate ATP hydrolysis by the mitochondrial F(1)-ATPase in the absence of γ. We found that the F446I mutation in the α subunit and G419D mutation in the β subunit suppress cell death by the loss of mitochondrial DNA (ρ(o)) in a Kluyveromyces lactis mutant lacking γ. In organello ATPase assays showed that the mutant but not the wild-type γ-less F(1) complexes retained 21.7 to 44.6% of the native F(1)-ATPase activity. The γ-less F(1) subcomplex was assembled but was structurally and functionally labile in vitro. Phe446 in the α subunit and Gly419 in the β subunit are located on the N-terminal edge of the DELSEED loops in both subunits. Mutations in these two sites likely enhance the transmission of catalytically required conformational changes to an adjacent α or β subunit, thereby allowing robust ATP hydrolysis and cell survival under ρ(o) conditions. This work may help our understanding of the structural elements required for ATP hydrolysis by the α(3)β(3) subcomplex.
Figures



References
-
- Ephrussi B, Hottinguer H, Tavlitzki J. 1949. Action de l'acriflavine sur les levures. II. Etude génétique du mutant “petite colonie”. Ann. Inst. Pasteur (Paris) 76:419–442
-
- Bulder CJ. 1964. Lethality of the petite mutation in petite negative yeasts. Antonie Van Leeuwenhoek 30:442–454 - PubMed
-
- Bulder CJ. 1964. Induction of petite mutation and inhibition of synthesis of respiratory enzymes in various yeasts. Antonie Van Leeuwenhoek 30:1–9 - PubMed
-
- Faye G, Fukuhara H, Grandchamp C, Lazowska J, Michel F, Casey J, Getz GS, Locker J, Rabinowitz M, Bolotin-Fukuhara M, Coen D, Deutsch J, Dujon B, Netter P, Slonimski PP. 1973. Mitochondrial nucleic acids in the petite colonie mutants: deletions and repetition of genes. Biochimie 55:779–792 - PubMed
-
- Chen XJ, Clark-Walker GD. 2000. The petite mutation in yeasts: 50 years on. Int. Rev. Cytol. 194:197–238 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

