MicroRNA-663 regulates human vascular smooth muscle cell phenotypic switch and vascular neointimal formation
- PMID: 24014830
- PMCID: PMC4537615
- DOI: 10.1161/CIRCRESAHA.113.301306
MicroRNA-663 regulates human vascular smooth muscle cell phenotypic switch and vascular neointimal formation
Abstract
Rationale: Abnormal phenotypic switch of vascular smooth muscle cell (VSMC) is a hallmark of vascular disorders such as atherosclerosis and restenosis after angioplasty. MicroRNAs (miRNAs) have emerged as important regulators for VSMC function, and we recently identified miR-663 as critical for controlling human aortic smooth muscle cell proliferation.
Objective: To investigate whether miR-663 plays a role in human VSMC phenotypic switch and the development of neointima formation.
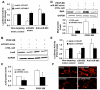
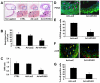
Methods and results: By using quantitative reverse-transcription polymerase chain reaction, we found that miR-663 was significantly downregulated in human aortic VSMCs on platelet-derived growth factor treatment, whereas expression was markedly increased during VSMC differentiation. Furthermore, we demonstrated that overexpression of miR-663 increased expression of VSMC differentiation marker genes, such as smooth muscle 22α, smooth muscle α-actin, calponin, and smooth muscle myosin heavy chain, and potently inhibited platelet-derived growth factor-induced VSMC proliferation and migration. We identified the transcription factor JunB and myosin light chain 9 as downstream targets of miR-663 in human VSMCs, because overexpression of miR-663 markedly inhibited expression of JunB and its downstream molecules, such as myosin light chain 9 and matrix metalloproteinase 9. Finally, we showed that adeno-miR-663 markedly suppressed the neointimal lesion formation by ≈50% in mice after vascular injury induced by carotid artery ligation, specifically via decreased JunB expression.
Conclusions: These results indicate that miR-663 is a novel modulator of human VSMC phenotypic switch by targeting JunB/myosin light chain 9 expression. These findings suggest that targeting miR-663 or its specific downstream targets in human VSMCs may represent an attractive approach for the treatment of proliferative vascular diseases.
Keywords: miR-663; migration; proliferation; vascular remodeling; vascular smooth muscle cells.
Figures
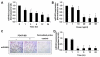
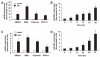
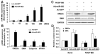
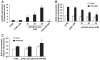


Comment in
-
miR-663 and the miRaculous vascular smooth muscle phenotypic switch.Circ Res. 2013 Oct 25;113(10):1102-5. doi: 10.1161/CIRCRESAHA.113.302578. Circ Res. 2013. PMID: 24158572 No abstract available.
References
-
- Rangrez AY, Massy ZA, Metzinger-Le Meuth V, Metzinger L. miR-143 and miR-145: molecular keys to switch the phenotype of vascular smooth muscle cells. Circ Cardiovasc Genet. 2011;4:197–205. - PubMed
-
- Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. - PubMed
-
- Tallquist M, Kazlauskas A. PDGF signaling in cells and mice. Cytokine Growth Factor Rev. 2004;15:205–213. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

