Evaluating Joint Effects of Induction-Salvage Treatment Regimes on Overall Survival in Acute Leukemia
- PMID: 24014891
- PMCID: PMC3762505
- DOI: 10.1111/j.1467-9876.2012.01048.x
Evaluating Joint Effects of Induction-Salvage Treatment Regimes on Overall Survival in Acute Leukemia
Abstract
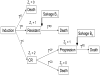
Typical oncology practice often includes not only an initial, frontline treatment, but also subsequent treatments given if the initial treatment fails. The physician chooses a treatment at each stage based on the patient's baseline covariates and history of previous treatments and outcomes. Such sequentially adaptive medical decision-making processes are known as dynamic treatment regimes, treatment policies, or multi-stage adaptive treatment strategies. Conventional analyses in terms of frontline treatments that ignore subsequent treatments may be misleading, because they actually are an evaluation of more than front-line treatment effects on outcome. We are motivated by data from a randomized trial of four combination chemotherapies given as frontline treatments to patients with acute leukemia. Most patients in the trial also received a second-line treatment, chosen adaptively and subjectively rather than by randomization, either because the initial treatment was ineffective or the patient's cancer later recurred. We evaluate effects on overall survival time of the 16 two-stage strategies that actually were used. Our methods include a likelihood-based regression approach in which the transition times of all possible multi-stage outcome paths are modeled, and estimating equations with inverse probability of treatment weighting to correct for bias. While the two approaches give different numerical estimates of mean survival time, they lead to the same substantive conclusions when comparing the two-stage regimes.
Keywords: Causal inference; Clinical trial; Dynamic treatment regime; Treatment policy.
Figures


References
-
- Estey EH, Thall PF, Pierce S, Cortes J, Beran M, Kantarjian H, Keating MJ, Andreeff M, Freireich E. Randomized phase II study of fludarabine + cytosine arabinoside + idarubicin ± granulocyte colony-stimulating factor in poor prognosis newly diagnosed acute myeloid leukemia and myelodysplastic syndrome. Blood. 1999;93(8):2478–2484. - PubMed
-
- Estey EH, Thall PF, Pierce S, Kantarjian H, Keating M. T Treatment of newly diagnosed acute promyelocytic leukemia without cytarabine. J Clinical Oncology. 1997;15:483–490. - PubMed
-
- Estey EH, Shen Y, Thall PF. Effect of time to complete remission on subsequent survival and disease-free survival in AML, RAEB-t, and RAEB. Blood. 2000;95(10):72–77. - PubMed
-
- Holland P. Statistics and causal inference. Journal of the American Statistical Association. 1986;81:945–960.
-
- Lavori P, Dawson R. A design for testing clinical strategies: biased individually tailored within-subject randomization. Journal of the Royal Statistical Society, A. 2000;163:29–38.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
