Retrospective evaluation of adverse transfusion reactions following blood product transfusion from a tertiary care hospital: A preliminary step towards hemovigilance
- PMID: 24014939
- PMCID: PMC3757769
- DOI: 10.4103/0973-6247.115564
Retrospective evaluation of adverse transfusion reactions following blood product transfusion from a tertiary care hospital: A preliminary step towards hemovigilance
Abstract
Background: The goal of hemovigilance is to increase the safety and quality of blood transfusion. Identification of the adverse reactions will help in taking appropriate steps to reduce their incidence and make blood transfusion process as safe as possible.
Aims: To determine the frequency and type of transfusion reactions (TRs) occurring in patients, reported to the blood bank at our institute.
Materials and methods: A retrospective review of all TRs reported to the blood bank at the All India Institute of Medical Sciences, between December 2007 and April 2012 was done. All the TRs were evaluated in the blood bank and classified using standard definitions.
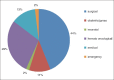
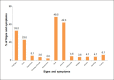
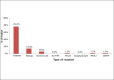
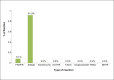
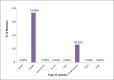
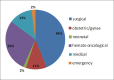
Results: During the study period a total of 380,658 bloods and blood components were issued by our blood bank. Out of the total 196 adverse reactions reported under the hemovigilance system, the most common type of reaction observed was allergic 55.1% (n = 108), followed by febrile non-hemolytic transfusion reaction (FNHTR) 35.7% (n = 70). Other less frequently observed reactions were Anaphylactoid reactions 5.1% (n = 10), Acute non-immune HTRs 2.6% (n = 5), Circulatory overload 0.5% (n = 1), Transfusion related acute lung injury 0.5% (n = 1), Delayed HTRs 0.5% (n = 1). Not a single case of bacterial contamination was observed.
Conclusion: The frequency of TRs in our patients was found to be 0.05% (196 out of 380,658). This can be an underestimation of the true incidence because of under reporting. It should be the responsibility of the blood transfusion consultant to create awareness amongst their clinical counterpart about safe transfusion practices so that proper hemovigilance system can be achieved to provide better patient care.
Keywords: Adverse transfusion reactions; blood transfusion; hemovigilance.
Conflict of interest statement
Figures


Similar articles
-
Transfusion-related adverse reactions: From institutional hemovigilance effort to National Hemovigilance program.Asian J Transfus Sci. 2016 Jan-Jun;10(1):31-6. doi: 10.4103/0973-6247.175391. Asian J Transfus Sci. 2016. PMID: 27011667 Free PMC article.
-
A Retrospective Analysis of Transfusion Reactions at a Tertiary Care Center: An Institutional Hemovigilance Study.J Contin Educ Nurs. 2021 Dec;52(12):581-588. doi: 10.3928/00220124-20211108-10. Epub 2021 Dec 1. J Contin Educ Nurs. 2021. PMID: 34870535
-
Patterns of Adverse Transfusion Reactions in a Tertiary Care Centre of North India: A Step Towards Hemovigilance.Indian J Hematol Blood Transfus. 2017 Jun;33(2):248-253. doi: 10.1007/s12288-016-0684-9. Epub 2016 May 24. Indian J Hematol Blood Transfus. 2017. PMID: 28596659 Free PMC article.
-
Noninfectious transfusion-associated adverse events and their mitigation strategies.Blood. 2019 Apr 25;133(17):1831-1839. doi: 10.1182/blood-2018-10-833988. Epub 2019 Feb 26. Blood. 2019. PMID: 30808635 Review.
-
Pulmonary transfusion reactions.Transfus Med Hemother. 2008 Oct;35(5):337-45. doi: 10.1159/000151349. Epub 2008 Sep 18. Transfus Med Hemother. 2008. PMID: 21512622 Free PMC article. Review.
Cited by
-
The effects of normovolemic anemia and blood transfusion on cerebral microcirculation after severe head injury.Intensive Care Med Exp. 2018 Nov 8;6(1):46. doi: 10.1186/s40635-018-0210-5. Intensive Care Med Exp. 2018. PMID: 30411308 Free PMC article.
-
Retrospective Audit of Transfusion Reactions in a Tertiary-Care Hospital in South India.Cureus. 2024 Dec 1;16(12):e74930. doi: 10.7759/cureus.74930. eCollection 2024 Dec. Cureus. 2024. PMID: 39744285 Free PMC article.
-
Incidence and Analysis of 7 Years Adverse Transfusion Reaction: A Retrospective Analysis.Indian J Hematol Blood Transfus. 2020 Jan;36(1):149-155. doi: 10.1007/s12288-019-01174-x. Epub 2019 Aug 30. Indian J Hematol Blood Transfus. 2020. PMID: 32158098 Free PMC article.
-
Active Hemovigilance Significantly Improves Reporting of Acute Non-infectious Adverse Reactions to Blood Transfusion.Indian J Hematol Blood Transfus. 2016 Sep;32(3):335-42. doi: 10.1007/s12288-015-0568-4. Epub 2015 Jul 16. Indian J Hematol Blood Transfus. 2016. PMID: 27429527 Free PMC article.
-
Pharmacovigilance in India: Present Scenario and Future Challenges.Drug Saf. 2019 Mar;42(3):339-346. doi: 10.1007/s40264-018-0730-7. Drug Saf. 2019. PMID: 30269244
References
-
- Sharma S, Sharma P, Tyler LN. Transfusion of blood and blood products: Indications and complications. Am Fam Physician. 2011;83:719–24. - PubMed
-
- Carson JL, Grossman BJ, Kleinman S, Tinmouth AT, Marques MB, Fung MK, et al. Red blood cell transfusion: A clinical practice guideline from the AABB*. Ann Intern Med. 2012;157:49–58. - PubMed
-
- Stainsby D, Jones H, Asher D, Atterbury C, Boncinelli A, Brant L, et al. Serious hazards of transfusion: A decade of hemovigilance in the UK. Transfus Med Rev. 2006;20:273–82. - PubMed
-
- Kleinman S, Chan P, Robillard P. Risks associated with transfusion of cellular blood components in Canada. Transfus Med Rev. 2003;17:120–62. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

