Comparative analysis of induced vs. spontaneous models of autoimmune uveitis targeting the interphotoreceptor retinoid binding protein
- PMID: 24015215
- PMCID: PMC3756070
- DOI: 10.1371/journal.pone.0072161
Comparative analysis of induced vs. spontaneous models of autoimmune uveitis targeting the interphotoreceptor retinoid binding protein
Abstract
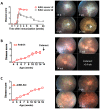
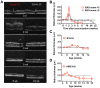
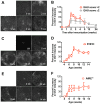
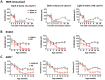
Animal models of autoimmunity to the retina mimic specific features of human uveitis, but no model by itself reproduces the full spectrum of human disease. We compared three mouse models of uveitis that target the interphotoreceptor retinoid binding protein (IRBP): (i) the "classical" model of experimental autoimmune uveitis (EAU) induced by immunization with IRBP; (ii) spontaneous uveitis in IRBP T cell receptor transgenic mice (R161H) and (iii) spontaneous uveitis in Autoimmune Regulator (AIRE)(-/-) mice. Disease course and severity, pathology and changes in visual function were studied using fundus imaging and histological examinations, optical coherence tomography and electroretinography. All models were on the B10.RIII background. Unlike previously reported, IRBP-induced EAU in B10.RIII mice exhibited two distinct patterns of disease depending on clinical scores developed after onset: severe monophasic with extensive destruction of the retina and rapid loss of visual signal, or lower grade with a prolonged chronic phase culminating after several months in retinal degeneration and loss of vision. R161H and AIRE(-/-) mice spontaneously developed chronic progressive inflammation; visual function declined gradually as retinal degeneration developed. Spontaneous uveitis in R161H mice was characterized by persistent cellular infiltrates and lymphoid aggregation, whereas AIRE(-/-) mice characteristically developed multi-focal infiltrates and severe choroidal inflammation. These data demonstrate variability and unique distinguishing features in the different models of uveitis, suggesting that each one can represent distinct aspects of uveitis in humans.
Conflict of interest statement
Figures



References
-
- Durrani OM, Meads CA, Murray PI (2004) Uveitis: a potentially blinding disease. Ophthalmologica 218: 223–236. - PubMed
-
- Gritz DC, Wong IG (2004) Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology 111: 491–500 discussion 500. - PubMed
-
- Nussenblatt RB WS (2004) Uveitis: fundamentals and clinical practice; edition r, editor. Philadelphia: Mosby (Elsevier).
-
- Caspi RR, Chan CC, Wiggert B, Chader GJ (1990) The mouse as a model of experimental autoimmune uveoretinitis (EAU). Curr Eye Res 9 Suppl: 169–174. - PubMed
-
- Forrester JV, Liversidge J, Dua HS, Dick A, Harper F, et al. (1992) Experimental autoimmune uveoretinitis: a model system for immunointervention: a review. Curr Eye Res 11 Suppl: 33–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

