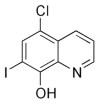
Clioquinol synergistically augments rescue by zinc supplementation in a mouse model of acrodermatitis enteropathica
- PMID: 24015258
- PMCID: PMC3755987
- DOI: 10.1371/journal.pone.0072543
Clioquinol synergistically augments rescue by zinc supplementation in a mouse model of acrodermatitis enteropathica
Abstract
Background: Zinc deficiency due to poor nutrition or genetic mutations in zinc transporters is a global health problem and approaches to providing effective dietary zinc supplementation while avoiding potential toxic side effects are needed.
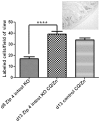
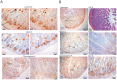
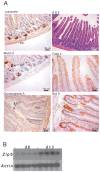
Methods/principal findings: Conditional knockout of the intestinal zinc transporter Zip4 (Slc39a4) in mice creates a model of the lethal human genetic disease acrodermatitis enteropathica (AE). This knockout leads to acute zinc deficiency resulting in rapid weight loss, disrupted intestine integrity and eventually lethality, and therefore provides a model system in which to examine novel approaches to zinc supplementation. We examined the efficacy of dietary clioquinol (CQ), a well characterized zinc chelator/ionophore, in rescuing the Zip4 (intest KO) phenotype. By 8 days after initiation of the knockout neither dietary CQ nor zinc supplementation in the drinking water was found to be effective at improving this phenotype. In contrast, dietary CQ in conjunction with zinc supplementation was highly effective. Dietary CQ with zinc supplementation rapidly restored intestine stem cell division and differentiation of secretory and the absorptive cells. These changes were accompanied by rapid growth and dramatically increased longevity in the majority of mice, as well as the apparent restoration of the homeostasis of several essential metals in the liver.
Conclusions: These studies suggest that oral CQ (or other 8-hydroxyquinolines) coupled with zinc supplementation could provide a facile approach toward treating zinc deficiency in humans by stimulating stem cell proliferation and differentiation of intestinal epithelial cells.
Conflict of interest statement
Figures



References
-
- Prasad AS (2012) Discovery of human zinc deficiency: 50 years later. J Trace Elem Med Biol 26: 66–69. - PubMed
-
- Neggers YH, Cutter GR, Acton RT, Alvarez JO, Bonner JL et al (1990) A positive association between maternal serum zinc concentration and birth weight. Am J Clin Nutr 51: 678–684. - PubMed
-
- Tamura T, Goldenberg RL, Johnston KE, DuBard M (2000) Maternal plasma zinc concentrations and pregnancy outcome. Am J Clin Nutr 71: 109–113. - PubMed
-
- Hambidge KM, Krebs NF (2003) Zinc, low birth weight, and breastfeeding. Pediatrics 112: 1419–1420. - PubMed
-
- Bloxam DL, Bax CMR (1996) Zinc deficiency and abnormal fetal development: Assessment of maternal or fetal zinc status. Am J Obstet Gynecol 175: 1078. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

