Optimization of isolated perfused/ventilated mouse lung to study hypoxic pulmonary vasoconstriction
- PMID: 24015341
- PMCID: PMC3757835
- DOI: 10.4103/2045-8932.114776
Optimization of isolated perfused/ventilated mouse lung to study hypoxic pulmonary vasoconstriction
Abstract
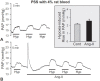
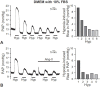
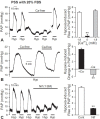
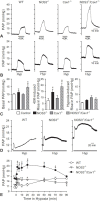
Hypoxic pulmonary vasoconstriction (HPV) is a compensatory physiological mechanism in the lung that optimizes the matching of ventilation to perfusion and thereby maximizes gas exchange. Historically, HPV has been primarily studied in isolated perfused/ventilated lungs; however, the results of these studies have varied greatly due to different experimental conditions and species. Therefore, in the present study, we utilized the mouse isolated perfused/ventilated lung model for investigation of the role of extracellular Ca(2+) and caveolin-1 and endothelial nitric oxide synthase expression on HPV. We also compared HPV using different perfusate solutions: Physiological salt solution (PSS) with albumin, Ficoll, rat blood, fetal bovine serum (FBS), or Dulbecco's Modified Eagle Medium (DMEM). After stabilization of the pulmonary arterial pressure (PAP), hypoxic (1% O2) and normoxic (21% O2) gases were applied via a ventilator in five-minute intervals to measure HPV. The addition of albumin or Ficoll with PSS did not induce persistent and strong HPV with or without a pretone agent. DMEM with the inclusion of FBS in the perfusate induced strong HPV in the first hypoxic challenge, but the HPV was neither persistent nor repetitive. PSS with rat blood only induced a small increase in HPV amplitude. Persistent and repetitive HPV occurred with PSS with 20% FBS as perfusate. HPV was significantly decreased by the removal of extracellular Ca(2+) along with addition of 1 mM EGTA to chelate residual Ca(2+) and voltage-dependent Ca(2+) channel blocker (nifedipine 1 μM). PAP was also reactive to contractile stimulation by high K(+) depolarization and U46619 (a stable analogue of thromboxane A2). In summary, optimal conditions for measuring HPV were established in the isolated perfused/ventilated mouse lung. Using this method, we further confirmed that HPV is dependent on Ca(2+) influx.
Keywords: endothelial nitric oxide synthase; hypoxia; mouse lung; pulmonary artery; pulmonary vasoconstriction.
Conflict of interest statement
Figures



Similar articles
-
Revisiting the mechanism of hypoxic pulmonary vasoconstriction using isolated perfused/ventilated mouse lung.Pulm Circ. 2020 Nov 25;10(4):2045894020956592. doi: 10.1177/2045894020956592. eCollection 2020 Oct-Dec. Pulm Circ. 2020. PMID: 33282184 Free PMC article.
-
Requirement of Pretone by Thromboxane A(2) for Hypoxic Pulmonary Vasoconstriction in Precision-cut Lung Slices of Rat.Korean J Physiol Pharmacol. 2012 Feb;16(1):59-64. doi: 10.4196/kjpp.2012.16.1.59. Epub 2012 Feb 28. Korean J Physiol Pharmacol. 2012. PMID: 22416221 Free PMC article.
-
Hypoxic pulmonary vasoconstriction: redox regulation of O2-sensitive K+ channels by a mitochondrial O2-sensor in resistance artery smooth muscle cells.J Mol Cell Cardiol. 2004 Dec;37(6):1119-36. doi: 10.1016/j.yjmcc.2004.09.007. J Mol Cell Cardiol. 2004. PMID: 15572043 Review.
-
Nitric oxide (NO)-dependent but not NO-independent guanylate cyclase activation attenuates hypoxic vasoconstriction in rabbit lungs.Am J Respir Cell Mol Biol. 2000 Aug;23(2):222-7. doi: 10.1165/ajrcmb.23.2.3935. Am J Respir Cell Mol Biol. 2000. PMID: 10919989
-
Integrative understanding of hypoxic pulmonary vasoconstriction using in vitro models: from ventilated/perfused lung to single arterial myocyte.Integr Med Res. 2014 Dec;3(4):180-188. doi: 10.1016/j.imr.2014.08.003. Epub 2014 Sep 3. Integr Med Res. 2014. PMID: 28664095 Free PMC article. Review.
Cited by
-
Mouse model of experimental pulmonary hypertension: Lung angiogram and right heart catheterization.Pulm Circ. 2021 Sep 8;11(4):20458940211041512. doi: 10.1177/20458940211041512. eCollection 2021 Oct-Dec. Pulm Circ. 2021. PMID: 34531976 Free PMC article.
-
Bacillus anthracis edema toxin inhibits hypoxic pulmonary vasoconstriction via edema factor and cAMP-mediated mechanisms in isolated perfused rat lungs.Am J Physiol Heart Circ Physiol. 2021 Jan 1;320(1):H36-H51. doi: 10.1152/ajpheart.00362.2020. Epub 2020 Oct 16. Am J Physiol Heart Circ Physiol. 2021. PMID: 33064559 Free PMC article.
-
Chloroquine is a potent pulmonary vasodilator that attenuates hypoxia-induced pulmonary hypertension.Br J Pharmacol. 2017 Nov;174(22):4155-4172. doi: 10.1111/bph.13990. Epub 2017 Oct 2. Br J Pharmacol. 2017. PMID: 28849593 Free PMC article.
-
TRPC6, a therapeutic target for pulmonary hypertension.Am J Physiol Lung Cell Mol Physiol. 2021 Dec 1;321(6):L1161-L1182. doi: 10.1152/ajplung.00159.2021. Epub 2021 Oct 27. Am J Physiol Lung Cell Mol Physiol. 2021. PMID: 34704831 Free PMC article.
-
Notch Activation of Ca(2+) Signaling in the Development of Hypoxic Pulmonary Vasoconstriction and Pulmonary Hypertension.Am J Respir Cell Mol Biol. 2015 Sep;53(3):355-67. doi: 10.1165/rcmb.2014-0235OC. Am J Respir Cell Mol Biol. 2015. PMID: 25569851 Free PMC article.
References
-
- von Euler US, Liljestrand G. Observations on the pulmonary arterial blood pressure in the cat. Acta Physiol Scand. 1946;12:301–20.
-
- Yuan JX, editor. Boston: Kluwer Academic Publishers; 2004. Hypoxic Pulmonary Vasoconstriction: Cellular and Molecular Mechanisms.
-
- Archer SL, Wu XC, Thébaud B, Nsair A, Bonnet S, Tyrrell B, et al. Preferential expression and function of voltage-gated, O2-sensitive K+ channels in resistance pulmonary arteries explains regional heterogeneity in hypoxic pulmonary vasoconstriction: Ionic diversity in smooth muscle cells. Circ Res. 2004;95:308–18. - PubMed
-
- Osipenko ON, Tate RJ, Gurney AM. Potential role for Kv3.1b channels as oxygen sensors. Circ Res. 2000;86:534–40. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

