Synthesis of a 2-aryl-3-aroyl indole salt (OXi8007) resembling combretastatin A-4 with application as a vascular disrupting agent
- PMID: 24016002
- PMCID: PMC3985392
- DOI: 10.1021/np400374w
Synthesis of a 2-aryl-3-aroyl indole salt (OXi8007) resembling combretastatin A-4 with application as a vascular disrupting agent
Abstract
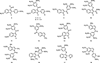
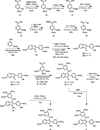
The natural products colchicine and combretastatin A-4 are potent inhibitors of tubulin assembly, and they have inspired the design and synthesis of a large number of small-molecule, potential anticancer agents. The indole-based molecular scaffold is prominent among these SAR modifications, leading to a rapidly increasing number of agents. The water-soluble phosphate prodrug 33 (OXi8007) of 2-aryl-3-aroylindole-based phenol 8 (OXi8006) was prepared by chemical synthesis and found to be strongly cytotoxic against selected human cancer cell lines (GI₅₀ = 36 nM against DU-145 cells, for example). The free phenol, 8 (OXi8006), was a strong inhibitor (IC₅₀ = 1.1 μM) of tubulin assembly. The corresponding phosphate prodrug 33 (OXi8007) also demonstrated pronounced interference with tumor vasculature in a preliminary in vivo study utilizing a SCID mouse model bearing an orthotopic PC-3 (prostate) tumor as imaged by color Doppler ultrasound. The combination of these results provides evidence that the indole-based phosphate prodrug 33 (OXi8007) functions as a vascular disrupting agent that may prove useful for the treatment of cancer.
Figures
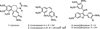


References
-
- Pinney KG, Pettit GR, Trawick ML, Jelinek C, Chaplin DJ. In: Antitumor Agents from Natural Products. 2nd ed. Cragg GM, Kingston DGI, Newman DJ, editors. Boca Raton, FL: CRC Press, Taylor and Francis Group; 2011. pp. 27–64.
-
- Hamel E. In: Microtubule Targets in Cancer Therapy. Fojo AT, editor. Totowa, NJ: Humana Press; 2008. pp. 1–20.
-
- Pettit GR, Cragg GM, Herald DL, Schmidt JM, Lohavanijaya P. Can. J. Chem. 1982;60:1374–1376.
-
- Pettit GR, Cragg GM, Singh SB. J. Nat. Prod. 1987;50:386–391. - PubMed
-
- Lin CM, Ho HH, Pettit GR, Hamel E. Biochemistry. 1989;28:6984–6991. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

